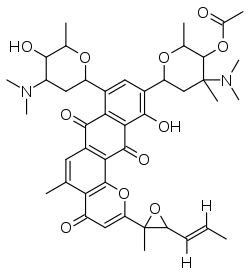
Pluramycin A
Pluramycin A is an antibiotic/anticancer compound that inhibits nucleic acid biosynthesis.[1] The pluramycin family of natural products are an important group of complex C-aryl glycoside antibiotics that possess the tetracyclic 4H-anthra[1,2-b]pyran-4,7,12-trione moiety A–D as an aromatic core. The D-ring is adorned with two deoxyaminosugars that are appended by C-aryl glycosidic linkages. The E-ring sugar is angolosamine, a carbohydrate that is also found in the antibiotic angolamycin. The F-ring sugar is the N,N-dimethyl derivative of vancosamine, which is the sugar found in the glycopeptide antibiotic vancomycin.
 | |
| Names | |
|---|---|
| IUPAC name
[4-(Dimethylamino)-6-[8-[4-(dimethylamino)-5-hydroxy-6-methyloxan-2-yl]-11-hydroxy-5-methyl-2-[2-methyl-3-[(E)-prop-1-enyl]oxiran-2-yl]-4,7,12-trioxonaphtho[2,3-h]chromen-10-yl]-2,4-dimethyloxan-3-yl] acetate | |
| Identifiers | |
PubChem CID |
|
| |
| Properties | |
| C43H52N2O11 | |
| Molar mass | 772.892 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
These compounds exhibit in vitro antitumor activity by DNA alkylation, where the two proximal amino sugars, D-angolosamine and N,N-dimethyl-L-vancosamine, play a key role in sequence recognition in intercalation of the tetracyclic chromophore.
References
- Nagai, K.; Yamaki, H.; Tanaka, N.; Umezwa, H. (1967). "Inhibition by pluramycin a of nucleic acid biosynthesis". Journal of Biochemistry. 62 (3): 321–7. PMID 4869973.