Platonic hydrocarbon
A Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed.[1]
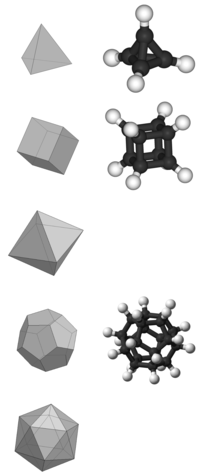
Not all Platonic solids have molecular hydrocarbon counterparts.
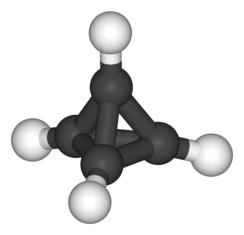
Tetrahedrane
Tetrahedrane (C4H4) is a hypothetical compound. It has not yet been synthesized without substituents, but it is predicted to be kinetically stable in spite of its angle strain. Some stable derivatives, including tetra(tert-butyl)tetrahedrane (a hydrocarbon) and tetra(trimethylsilyl)tetrahedrane, have been produced.
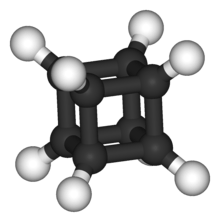
Cubane
Cubane (C8H8) has been synthesized. Although it has high angle strain, cubane is kinetically stable, due to a lack of readily available decomposition paths.
Octahedrane
Angle strain would make an octahedron highly unstable due to inverted tetrahedral geometry at each vertex. There would also be no hydrogen atoms because four edges meet at each corner; thus, the hypothetical octahedrane molecule would be an allotrope of elemental carbon, C6, and not a hydrocarbon. The existence of octahedrane cannot be ruled out completely, although calculations have shown that it is unlikely.[2]
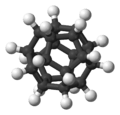
Dodecahedrane
Dodecahedrane (C20H20) was first synthesized in 1982, and has almost zero angle strain thanks to its angles of 108°, which are close to the tetrahedral angle of 109.5°.[3]
Icosahedrane
The tetravalency (4-connectedness) of carbon excludes an icosahedron because 5 edges meet at each vertex. True pentavalent carbon is unlikely; methanium, nominally CH+
5, usually exists as CH
3(H
2)+
. The hypothetical icosahedral C12+
12 lacks hydrogen so it is not a hydrocarbon; it is also an ion.
Both icosahedral and octahedral structures have been observed in boron compounds[2] such as the dodecaborate ion and some of the carbon-containing carboranes.
Other polyhedra
Increasing the number of atoms that comprise the carbon skeleton leads to a geometry that increasingly approximates a sphere, and the space enclosed in the carbon "cage" increases. This trend continues with buckyballs or spherical fullerene (C60). Although not a Platonic hydrocarbon, buckminsterfullerene has the shape of a truncated icosahedron, an Archimedean solid.
The concept can also be extended to regular Euclidean tilings, with the hexagonal tiling producing graphane. A square tiling (which would resemble an infinitely large fenestrane) would suffer form the same problem as octahedrane, and the triangular tiling icosahedrane. No generalisations to hyperbolic tilings seem to be known.
The regular convex 4-polytopes may also have hydrocarbon analogues; hypercubane has been proposed.
Gallery
References
- Henning Hopf, Classics in Hydrocarbon Chemistry, Wiley VCH, 2000.
- Lewars, Errol G. (2008). Modeling Marvels: Computational Anticipation of Novel molecules. Springer Science+Business Media. pp. 81–82. doi:10.1007/978-1-4020-6973-4. ISBN 978-1-4020-6972-7. Retrieved January 30, 2012.
- Ternansky, Robert J.; Balogh, Douglas W.; Paquette, Leo A. (1982). "Dodecahedrane". J. Am. Chem. Soc. 104 (16): 4503–4504. doi:10.1021/ja00380a040.
| Wikimedia Commons has media related to Platonic hydrocarbons. |