Phenoxathiin
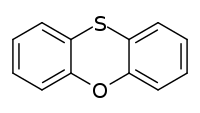
Phenoxathiin (dibenzooxathiane) C12H8OS is a heterocyclic compound of molecular weight 200.25632 g/mol with the CAS Registry Number 262-20-4.[2]
 | |
| Names | |
|---|---|
| IUPAC name
Phenoxathiine | |
| Other names
1,4-Dibenzothioxine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.433 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H8OS | |
| Molar mass | 200.26 g·mol−1 |
| Melting point | 52–56 °C (126–133 °F; 325–329 K)[1] |
| Boiling point | 150–152 °C (302–306 °F; 423–425 K)[1] (at 5 mmHg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diphenyl ether is a starting material in the production of phenoxathiin via the Ferrario reaction.[3] Phenoxathiin is used in polyamide and polyimide production.[4]
References
- "Phenoxathiin". Sigma-Aldrich.
- The Chemistry of Phenoxathiin and its Derivatives. Clara L. Deasy Chem. Rev., 1943, 32 (2), pp 173–194 DOI: 10.1021/cr60102a001 Publication Date: April 1943
- Suter, C. M.; Maxwell, C. E. (1943). "Phenoxthin". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 2, p. 485
- Mitsuru Ueoda; Tatsuo Aizawa; Yoshio Imai (1977). "Preparation and properties of polyamides and polyimides containing phenoxathiin units". Journal of Polymer Science: Polymer Chemistry Edition. 15 (11): 2739–2747. doi:10.1002/pol.1977.170151119.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.