Diphenyl ether
Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless solid. This, the simplest diaryl ether, has a variety of niche applications.[5]
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1'-Oxydibenzene[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| 1364620 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.711 |
| EC Number |
|
| 165477 | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H10O | |
| Molar mass | 170.211 g·mol−1 |
| Appearance | Colorless solid or liquid |
| Odor | geranium-like |
| Density | 1.08 g/cm3 (20°C)[2] |
| Melting point | 25 to 26 °C (77 to 79 °F; 298 to 299 K) |
| Boiling point | 121 °C (250 °F; 394 K)[3] at 1.34 kPa (10.05 mm Hg), 258.55 °C at 100 kPa (1 bar) |
| Insoluble | |
| Vapor pressure | 0.02 mmHg (25°C)[2] |
| -108.1·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | Aldrich MSDS |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H319, H400, H411 |
| P264, P273, P280, P305+351+338, P337+313, P391, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 115 °C (239 °F; 388 K) |
| Explosive limits | 0.7%-6.0%[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
3370 mg/kg (rat, oral) 4000 mg/kg (rat, oral) 4000 mg/kg (guinea pig, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1 ppm (7 mg/m3)[2] |
REL (Recommended) |
TWA 1 ppm (7 mg/m3)[2] |
IDLH (Immediate danger) |
100 ppm[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
Diphenyl ether and many of its properties were first reported as early as 1901.[6] It is synthesized by a modification of the Williamson ether synthesis, here the reaction of phenol and bromobenzene in the presence of base and a catalytic amount of copper:
- PhONa + PhBr → PhOPh + NaBr
Involving similar reactions, diphenyl ether is a significant side product in the high-pressure hydrolysis of chlorobenzene in the production of phenol.[7]
Related compounds are prepared by Ullmann reactions.[8]
The compound undergoes reactions typical of other phenyl rings, including hydroxylation, nitration, halogenation, sulfonation, and Friedel–Crafts alkylation or acylation.[5]
Uses
The main application of diphenyl ether is as a eutectic mixture with biphenyl, used as a heat transfer medium. Such a mixture is well-suited for heat transfer applications because of the relatively large temperature range of its liquid state. A eutectic mixture [commercially, Dowtherm A] is 73.5% diphenyl ether (diphenyl oxide) and 26.5% biphenyl (diphenyl).[9][10]
Diphenyl ether is a starting material in the production of phenoxathiin via the Ferrario reaction.[11] Phenoxathiin is used in polyamide and polyimide production.[12]
Because of its odor reminiscent of scented geranium, as well as its stability and low price, diphenyl ether is used widely in soap perfumes. Diphenyl ether is also used as a processing aid in the production of polyesters.[5]
Related compounds
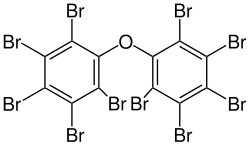
Several polybrominated diphenyl ethers (PBDEs) are useful flame retardants. Of penta-, octa-, and decaBDE, the three most common PBDEs, only decaBDE is still in widespread use since its ban in the European Union in 2003.[13] DecaBDE, also known as decabromodiphenyl oxide,[14] is a high-volume industrial chemical with over 450,000 kilograms produced annually in the United States. Decabromodiphenyl oxide is sold under the trade name Saytex 102 as a flame retardant in the manufacture of paints and reinforced plastics.
References
- "CHAPTER P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 705. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0496". National Institute for Occupational Safety and Health (NIOSH).
- Byers, Charles H.; Williams, David F. (July 1987). "Viscosities of pure polyaromatic hydrocarbons". Journal of Chemical & Engineering Data. 32 (3): 344–348. doi:10.1021/je00049a018. ISSN 0021-9568.
- "Phenyl ether". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- Cook, A. N. (1901). "Derivatives of Phenylether". Journal of the American Chemical Society. 23 (10): 806–813. doi:10.1021/ja02037a005.
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. (2003). Flavor and Fragrances. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
- Ungnade, H. E.; Orwoll, E. F. (1946). "2-Methoxy Diphenyl Ether". Org. Synth. 26: 50. doi:10.15227/orgsyn.026.0050.
- Patent Appeal No. 7555 United States Court of Customs and Patent Appeals 7 April 1966 http://openjurist.org/358/f2d/750/application-of-edward-s-blake-and-william-c-hammann
- "Dowtherm® A 44570".
- Suter, C. M.; Maxwell, C. E. (1943). "Phenoxthin". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 2, p. 485
- Mitsuru Ueoda; Tatsuo Aizawa; Yoshio Imai (1977). "Preparation and properties of polyamides and polyimides containing phenoxathiin units". Journal of Polymer Science: Polymer Chemistry Edition. 15 (11): 2739–2747. doi:10.1002/pol.1977.170151119.
- DIRECTIVE 2003/11/EC of the European Parliament and of the Council
- Sutker, B. J. (2005). Flame Retardants. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_123. ISBN 978-3-527-30673-2.
