Periostin
Periostin (POSTN, PN, or osteoblast-specific factor OSF-2) is a protein that in humans is encoded by the POSTN gene.[5][6] Periostin functions as a ligand for alpha-V/beta-3 and alpha-V/beta-5 integrins to support adhesion and migration of epithelial cells.[7]
Periostin is a gla domain vitamin K dependent factor.[8]
Function
Periostin is a secreted extracellular matrix protein that was originally identified in cells from the mesenchymal lineage (osteoblasts, osteoblast-derived cells, the periodontal ligament, and periosteum). It has been associated with the epithelial-mesenchymal transition in cancer and with the differentiation of mesenchyme in the developing heart. This protein shares a homology with fasciclin I, a secreted cell adhesion molecule found in insects.
In many cancers, periostin binds to integrins on cancer cells, activating the Akt/PKB- and FAK-mediated signaling pathways. This leads to increased cell survival, invasion, angiogenesis, metastasis, and the epithelial-mesenchymal transition.[10]
In humans and mice, periostin undergoes alternative splicing in its C-terminal region, resulting in specific isoforms that can be observed in a broad range of cancers such as pancreatic, colon, and breast cancer.
While periostin plays a wide variety of roles in tissue development along with disease, its function in tissue remodeling as a response to injury is a common underlying role in these different mechanisms. Periostin is transiently upregulated during cell fate changes, whether they are related to alterations in physiology or to pathological changes. It influences extracellular matrix restructuring, tissue remodeling, and the epithelial-mesenchymal transition, all of which can be related to tissue healing, development, and disease. Thus, it functions as a mediator, balancing appropriate and inappropriate responses to tissue damage.[11]
Clinical significance
In valvular heart disease
Periostin plays a critical role in the development of cardiac valves and in degenerative valvular heart disease. While periostin usually is localized to the subendothelial layer in healthy heart valves, its levels are highly increased in infiltrated inflammatory cells and myofibroblasts in angiogenic areas in atherosclerotic and rheumatic valvular heart disease in humans. Periostin has also been shown to increase the secretion of matrix metalloproteinase from valvular intestinal cells, endothelial cells, and macrophages. It is thought that periostin plays a role in cardiac valve complex degeneration by inducing both angiogenesis and matrix metalloproteinase production.[12]
In tissue regeneration and healing
As a matricellular protein, periostin is also important for tissue regeneration. In healthy human skin, periostin is expressed at basal levels and is expressed in the epidermis and hair follicles along with fibronectin and laminin γ2.[11][13] Periostin is involved in wound healing, helping for the wound to heal faster than when periostin is not present in cells. This delay in wound closure is also associated with a delay in re-epithelialization and a reduction in the proliferation of keratinocytes.[13] Periostin localizes to the extracellular compartment of cells during tissue remodeling associated with wound repair. It may also promote injury closure by facilitating the activation, differentiation, and contraction of fibroblasts. However, the increase in periostin expression associated with tissue regeneration post-injury is transient, starting a few days post-injury, peaking after seven days post-injury, and decreasing afterwards.[11]
In asthma
Periostin is associated with asthma, a fact that is exploited by the experimental asthma medication lebrikizumab.[14]
In cancer
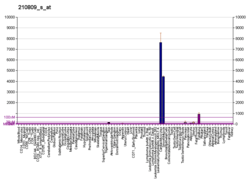
Periostin over-expression was reported in several types of cancer, most frequently in the environment of tumor cells.[7][15] Recent evidence shows that periostin is a component of the extracellular matrix expressed by fibroblasts in normal tissues and stroma of primary tumor. The metastatic colony formation requires the induction of periostin in the foreign stroma by the infiltrating cancer cells. Periostin production is upregulated in lung fibroblasts by either TGF-β2 or TGF-β3, the latter being secreted by infiltrating cancer stem cells (in MMTV-PyMT mouse breast cancer model) [16]
Periostin has been shown to be highly upregulated in glioblastomas (grade IV gliomas) compared to the normal brain. In gliomas, periostin expression levels correlate directly with tumor grade and recurrence, and inversely with survival.[17] It has been shown that glioma stem cells in glioblastomas secrete periostin, which recruits M2 tumor-associated macrophages from peripheral blood to the tumor environment via αvβ3 integrin signaling. These M2 TAMs differentiate from monocytes once they enter the tumor tissue. Through this recruitment mechanism, periostin supports tumor progression, as M2 tumor-associated macrophages are tumor-supportive and immunosuppressive. In this environment, periostin functions as a chemoattractant, promoting both migration and invasion of macrophages and monocytes into glioblastomas in a dose-dependent manner.[18] Clinically, periostin-associated gene signatures, which are predominated by secreted and matrix proteins, correspond to patient prognosis and malignancy. Given its features related to glioblastoma progression, periostin is a marker of glioma malignancy as well as recurrence of tumors, making it a possible target for therapy that continues to be studied and explored.[17]
Table: Periostin expression in various cancer cell lines.[19]
| Cell line | Origin | POSTN/ACTB1 |
|---|---|---|
| U2OS | Osteosarcoma | 3.5±1.7 |
| LB96 | Ewing Sarcoma | 0 |
| LB23-1 | Rhabdomyosarcoma | 0.1±0.1 |
| HeLa | Cervical cancer | 3.0±0.4 |
| PA-1 | Ovarian teratocarcinoma | 1.4±0.1 |
| LB37-1 | NSCLC | |
| LB85 | SCLC | 3.4±0.2 |
| LB92 | SCLC | 0.6±0.2 |
| LB1047 | Renal cell carcinoma | 0.8±0.2 |
| BB64 | Renal cell carcinoma | 0.08±0.01 |
| LB108 | Colorectal cancer | 0 |
| MCF7 | Breast Cancer | 0 |
| Hs578T | Breast Cancer | 3693±86 |
| Panc-1 | Pancreatic carcinoma | 0 |
| Capan-1 | Pancreatic carcinoma | 0 |
| Huh-7 | Hepatocarcinoma | 0.3±0.07 |
| LB831 | Bladder carcinoma | 1748±74 |
| MZGC3 | Stomach cancer | 0 |
| A172 | Glioblastoma | 45±4 |
| MZ2 | Melanoma | 2.3±0.7 |
| LB39 | Melanoma | 0.5±0.03 |
| LB2586-7 | Melanoma | 3.4±0.3 |
| LB2201-3 | Melanoma | 4.2±0.4 |
| A375 | Melanoma | 4.7±1.2 |
1 (cDNA POSTN/cDNA ACTB) × 104
References
- GRCh38: Ensembl release 89: ENSG00000133110 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027750 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Takeshita S, Kikuno R, Tezuka K, Amann E (Aug 1993). "Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I". The Biochemical Journal. 294. 294 (1): 271–8. doi:10.1042/bj2940271. PMC 1134594. PMID 8363580.
- "Entrez Gene: POSTN periostin, osteoblast specific factor".
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD (Sep 2002). "Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility". Cancer Research. 62 (18): 5358–64. PMID 12235007.
- Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J (Jun 2008). "Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells". The Journal of Biological Chemistry. 283 (26): 17991–8001. doi:10.1074/jbc.M708029200. PMID 18450759.
- Morra L, Moch H (Nov 2011). "Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update". Virchows Archiv. 459 (5): 465–75. doi:10.1007/s00428-011-1151-5. PMC 3205268. PMID 21997759.
- Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holweg CT, Kudo A (Apr 2014). "The role of periostin in tissue remodeling across health and disease". Cellular and Molecular Life Sciences. 71 (7): 1279–88. doi:10.1007/s00018-013-1494-y. PMC 3949008. PMID 24146092.
- Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K (Jul 2010). "Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents". The Journal of Clinical Investigation. 120 (7): 2292–306. doi:10.1172/JCI40973. PMC 2898587. PMID 20551517.
- Braun N, Sen K, Alscher MD, Fritz P, Kimmel M, Morelle J, Goffin E, Jörres A, Wüthrich RP, Cohen CD, Segerer S (2013). "Periostin: a matricellular protein involved in peritoneal injury during peritoneal dialysis". Peritoneal Dialysis International. 33 (5): 515–28. doi:10.3747/pdi.2010.00259. PMC 3797670. PMID 23378472.
- Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG (Sep 2011). "Lebrikizumab treatment in adults with asthma". The New England Journal of Medicine. 365 (12): 1088–98. doi:10.1056/NEJMoa1106469. PMID 21812663.
- Contié S, Voorzanger-Rousselot N, Litvin J, Clézardin P, Garnero P (Jan 2011). "Increased expression and serum levels of the stromal cell-secreted protein periostin in breast cancer bone metastases". International Journal of Cancer. 128 (2): 352–60. doi:10.1002/ijc.25591. PMID 20715172.
- Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (Jan 2012). "Interactions between cancer stem cells and their niche govern metastatic colonization". Nature. 481 (7379): 85–9. doi:10.1038/nature10694. PMID 22158103.
- Mikheev AM, Mikheeva SA, Trister AD, Tokita MJ, Emerson SN, Parada CA, Born DE, Carnemolla B, Frankel S, Kim DH, Oxford RG, Kosai Y, Tozer-Fink KR, Manning TC, Silber JR, Rostomily RC (Aug 2014). "Periostin is a novel therapeutic target that predicts and regulates glioma malignancy". Neuro-Oncology. 17 (3): 372–82. doi:10.1093/neuonc/nou161. PMC 4483094. PMID 25140038.
- Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S (Feb 2015). "Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth". Nature Cell Biology. 17 (2): 170–82. doi:10.1038/ncb3090. PMC 4312504. PMID 25580734.
- Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A (2007). "Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells". Molecular Cancer. 6 (80): 80. doi:10.1186/1476-4598-6-80. PMC 2222651. PMID 18086302.
Further reading
- Sasaki H, Dai M, Auclair D, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y, Chen LB (Aug 2001). "Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas". Cancer. 92 (4): 843–8. doi:10.1002/1097-0142(20010815)92:4<843::AID-CNCR1391>3.0.CO;2-P. PMID 11550156.
- Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF (May 2004). "Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression". Molecular and Cellular Biology. 24 (9): 3992–4003. doi:10.1128/MCB.24.9.3992-4003.2004. PMC 387763. PMID 15082792.
- Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF (Apr 2004). "Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway". Cancer Cell. 5 (4): 329–39. doi:10.1016/S1535-6108(04)00081-9. PMID 15093540.
- Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H (Oct 2005). "Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells". International Journal of Cancer. 117 (1): 51–8. doi:10.1002/ijc.21120. PMID 15880581.
- Chang Y, Lee TC, Li JC, Lai TL, Chua HH, Chen CL, Doong SL, Chou CK, Sheen TS, Tsai CH (Oct 2005). "Differential expression of osteoblast-specific factor 2 and polymeric immunoglobulin receptor genes in nasopharyngeal carcinoma". Head & Neck. 27 (10): 873–82. doi:10.1002/hed.20253. PMID 16136586.
- Liu T, Qian WJ, Gritsenko MA, Camp DG, Monroe ME, Moore RJ, Smith RD (2006). "Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry". Journal of Proteome Research. 4 (6): 2070–80. doi:10.1021/pr0502065. PMC 1850943. PMID 16335952.
- Yan W, Shao R (Jul 2006). "Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation". The Journal of Biological Chemistry. 281 (28): 19700–8. doi:10.1074/jbc.M601856200. PMID 16702213.
- Försti A, Jin Q, Altieri A, Johansson R, Wagner K, Enquist K, Grzybowska E, Pamula J, Pekala W, Hallmans G, Lenner P, Hemminki K (Jan 2007). "Polymorphisms in the KDR and POSTN genes: association with breast cancer susceptibility and prognosis". Breast Cancer Research and Treatment. 101 (1): 83–93. doi:10.1007/s10549-006-9265-1. PMID 16807673.
- Grigoriadis A, Mackay A, Reis-Filho JS, Steele D, Iseli C, Stevenson BJ, Jongeneel CV, Valgeirsson H, Fenwick K, Iravani M, Leao M, Simpson AJ, Strausberg RL, Jat PS, Ashworth A, Neville AM, O'Hare MJ (2007). "Establishment of the epithelial-specific transcriptome of normal and malignant human breast cells based on MPSS and array expression data". Breast Cancer Research. 8 (5): R56. doi:10.1186/bcr1604. PMC 1779497. PMID 17014703.
- Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR (Mar 2007). "Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway". Oncogene. 26 (14): 2082–94. doi:10.1038/sj.onc.1210009. PMID 17043657.
- Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T (Nov 2006). "Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer". British Journal of Cancer. 95 (10): 1396–403. doi:10.1038/sj.bjc.6603431. PMC 2360586. PMID 17060937.
- Li JS, Sun GW, Wei XY, Tang WH (Oct 2007). "Expression of periostin and its clinicopathological relevance in gastric cancer". World Journal of Gastroenterology. 13 (39): 5261–6. doi:10.3748/wjg.v13.i39.5261. PMC 4171309. PMID 17876898.
- Contié S, Voorzanger-Rousselot N, Litvin J, Bonnet N, Ferrari S, Clézardin P, Garnero P (Oct 2010). "Development of a new ELISA for serum periostin: evaluation of growth-related changes and bisphosphonate treatment in mice". Calcified Tissue International. 87 (4): 341–50. doi:10.1007/s00223-010-9391-y. PMID 20567965.
- Kashyap MK, Marimuthu A, Peri S, Kumar GS, Jacob HK, Prasad TS, Mahmood R, Kumar KV, Kumar MV, Meltzer SJ, Montgomery EA, Kumar RV, Pandey A (2010). "Overexpression of periostin and lumican in esophageal squamous cell carcinoma". Cancers. 2 (1): 133–42. doi:10.3390/cancers2010133. PMC 3827595. PMID 24281036.