Partial melting
Partial melting occurs when only a portion of a solid is melted. For mixed substances, such as a rock containing several different minerals or a mineral that displays solid solution, this melt can be different from the bulk composition of the solid. Partial melting occurs where the solidus and liquidus temperatures are different. For single minerals this can happen when they exhibit solid solution, for example in olivines between iron and magnesium. In rocks made up of several different minerals, some will melt at lower temperatures than others.
Partial melting of the mantle
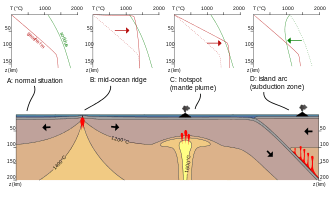
Melting in the mantle requires one of three possible events to occur: an increase in temperature, a decrease in pressure, or the addition of volatiles to the system (a change in composition).[1]
Temperature
In the case of raising the temperature, mantle melting will only occur if the mantle is heated past the normal geotherm. It is believed that heat flux from the core and lower mantle is responsible for increasing the temperature of the upper mantle. Local perturbations of the geothermal gradient, such as hotspots, are not well understood but are considered to be a likely heat source for the mantle.The decay of radioactive elements, though considered to be one of the simplest ways of generating heat in the mantle, is not realistically responsible for mantle melting, as it would take over 10 million years for the radioactive decay of K, U and Th to increase the temperature of peridotite by 1 degree Celsius. Furthermore, even if this process did generate a small fraction of melt, the radioactive elements would concentrate in the melt and escape the system, ultimately halting the process of melt generation.[1]
Pressure

Melting in the mantle can also occur if there is a sufficient drop in pressure in the system at a given temperature. In order to decrease pressure, mantle rocks must rise to shallower levels, while experiencing a minimal loss of heat to the surroundings. This process can be referred to as adiabatic if the heat loss is zero. As the mass of mantle rock continues to rise through the Earth's layers, it follows a P-T path that may eventually cross the solidus, initiating melting. This melting process is known as decompression melting.[1]
Addition of volatiles
The presence of volatiles (particularly H2O and CO2) has the potential to significantly reduce solidus temperatures of a given system. This allows for melt to be generated at lower temperatures than otherwise predicted, eliminating the need for a change in pressure or temperature conditions of the system. However, the mantle typically has a very low volatile content and this can limit the amount of melt generated. Partial melting is an important process in geology with respect to the chemical differentiation of crustal rocks. On the Earth, partial melting of the mantle at mid-ocean ridges produces oceanic crust, and partial melting of the mantle and oceanic crust at subduction zones creates continental crust. In all these places partial melting is often associated with volcanism, although some melts do not make it to the surface. Partial melts are thought to play an important role in enriching old parts of the continental lithosphere in incompatible elements.[2] Partial melts produced at depth move upwards due to the compaction of the surrounding matrix.[1]
References
- Winter, John D. (John DuNann) (2015). Principles of igneous and metamorphic petrology. Pearson India Education Services. ISBN 9789332550407. OCLC 931961923.
- Gibson, Sally A.; Jacqueline Malarkey; Jason A. Day (2008-10-22). "Melt Depletion and Enrichment beneath the Western Kaapvaal Craton: Evidence from Finsch Peridotite Xenoliths". Journal of Petrology. 49 (10): egn048. doi:10.1093/petrology/egn048. Archived from the original on 2012-07-17. Retrieved 2009-05-22.