4-Nitroaniline
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. It is an organic chemical compound, consisting of a benzene ring in which an amino group is para to a nitro group. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals, gasoline, gum inhibitors, poultry medicines, and as a corrosion inhibitor.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Nitroaniline | |||
| Systematic IUPAC name
4-Nitrobenzenamine | |||
| Other names
p-Nitroaniline 1-Amino-4-nitrobenzene p-Nitrophenylamine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 508690 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.555 | ||
| EC Number |
| ||
| 27331 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1661 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H6N2O2 | |||
| Molar mass | 138.12 g/mol | ||
| Appearance | yellow or brown powder | ||
| Odor | faint, ammonia-like | ||
| Density | 1.437 g/ml, solid | ||
| Melting point | 146 to 149 °C (295 to 300 °F; 419 to 422 K) (lit.) | ||
| Boiling point | 332 °C (630 °F; 605 K) | ||
| 0.8 mg/ml at 18.5 °C (IPCS) | |||
| Vapor pressure | 0.00002 mmHg (20°C)[1] | ||
| -66.43·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Toxic | ||
| Safety data sheet | JT Baker | ||
| GHS pictograms |   | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H301, H311, H331, H373, H412 | ||
| P260, P261, P264, P270, P271, P273, P280, P301+310, P302+352, P304+340, P311, P312, P314, P321, P322, P330, P361, P363, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 199 °C (390 °F; 472 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
3249 mg/kg (rat, oral) 750 mg/kg (rat, oral) 450 mg/kg (guinea pig, oral) 810 mg/kg (mouse, oral)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 6 mg/m3 (1 ppm) [skin][1] | ||
REL (Recommended) |
TWA 3 mg/m3 [skin][1] | ||
IDLH (Immediate danger) |
300 mg/m3[1] | ||
| Related compounds | |||
Related compounds |
2-Nitroaniline, 3-Nitroaniline | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
It is produced industrially via the amination of 4-nitrochlorobenzene:[3]
- ClC6H4NO2 + 2 NH3 → H2NC6H4NO2 + NH4Cl
Below is a laboratory synthesis of 4-nitroaniline from aniline. The key step in this reaction sequence is an electrophilic aromatic substitution to install the nitro group para to the amino group. The amino group can be easily protonated and become a meta director. Therefore, a protection of the acetyl group is required. After this reaction, a separation must be performed to remove 2-nitroaniline, which is also formed in a small amount during the reaction.[4]
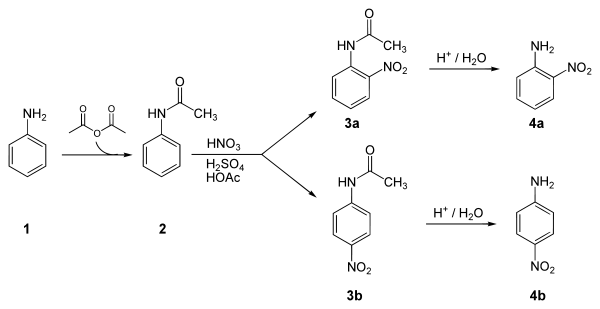
Applications
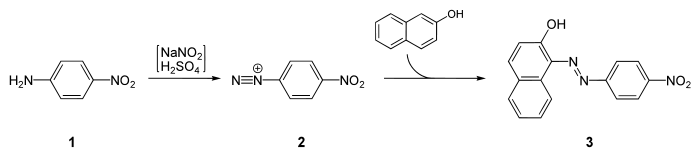
4-Nitroaniline is mainly consumed industrially as a precursor to p-phenylenediamine, an important dye component. The reduction is effected using iron metal and by catalytic hydrogenation.[3]
It is a starting material for the synthesis of Para Red, the first azo dye:[5]
When heated with sulfuric acid, it polymerizes explosively into a rigid foam.[6]
Laboratory use
Nitroaniline is a solvatochromic dye used for determining Kamlet-Taft solvent parameters. The position of its UV-visual peak changes with the balance of hydrogen bonding acceptors and donors in the solvent.
Toxicity
The compound is toxic by way of inhalation, ingestion, and absorption, and should be handled with care. Its LD50 in rats is 750 mg/kg when administered orally. 4-Nitroaniline is particularly harmful to all aquatic organisms, and can cause long-term damage to the environment if released as a pollutant.
See also
References
- NIOSH Pocket Guide to Chemical Hazards. "#0449". National Institute for Occupational Safety and Health (NIOSH).
- "p-Nitroaniline". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Gerald Booth (2007). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
- Mohrig, J.R.; Morrill, T.C.; Hammond, C.N.; Neckers, D.C. (1997). "Synthesis 5: Synthesis of the Dye Para Red from Aniline". Experimental Organic Chemistry. New York, NY: Freeman. pp. 456–467.
- Williamson, Kenneth L. (2002). Macroscale and Microscale Organic Experiments, Fourth Edition. Houghton-Mifflin. ISBN 0-618-19702-8.
- Poshkus, A. C.; Parker, J. A. (1970). "Studies on nitroaniline–sulfuric acid compositions: Aphrogenic pyrostats". Journal of Applied Polymer Science. 14 (8): 2049–2064. doi:10.1002/app.1970.070140813.