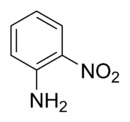
2-Nitroaniline
2-Nitroaniline is an organic compound with the formula H2NC6H4NO2. It is a derivative of aniline, carrying a nitro functional group in position 2.[1] It is mainly used as a precursor to o-phenylenediamine.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Nitroaniline | |||
| Systematic IUPAC name
2-Nitrobenzenamine | |||
| Other names
ortho-Nitroaniline o-Nitroaniline | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.687 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1661 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H6N2O2 | |||
| Molar mass | 138.126 g·mol−1 | ||
| Appearance | Orange solid | ||
| Density | 1.442 g/mol | ||
| Melting point | 71.5 °C (160.7 °F; 344.6 K) | ||
| Boiling point | 284 °C (543 °F; 557 K) | ||
| 0.117 g/100 ml (20°C) (SIDS) | |||
| Acidity (pKa) | -0.3 (of anilinium salt) | ||
| -66.47·10−6 cm3/mol | |||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H301, H311, H331, H373, H412 | ||
| P260, P261, P264, P270, P271, P273, P280, P301+310, P302+352, P304+340, P311, P312, P314, P321, P322, P330, P361, P363, P403+233, P405, P501 | |||
| Flash point | 168 °C (334 °F; 441 K) | ||
| Related compounds | |||
Related compounds |
3-Nitroaniline, 4-Nitroaniline | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
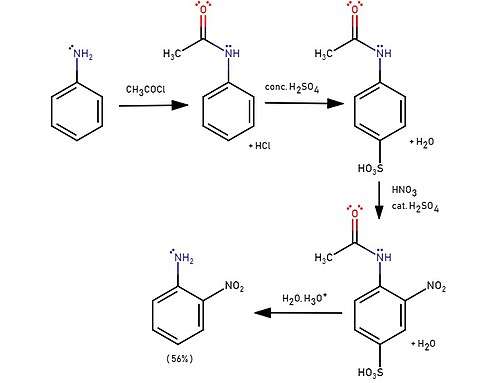
Synthesis
2-Nitroaniline is prepared commercially by the reaction of 2-nitrochlorobenzene with ammonia:[2]
- ClC6H4NO2 + 2 NH3 → H2NC6H4NO2 + NH4Cl
Many other methods exist for the synthesis of this compound. Direct nitration of aniline is inefficient since anilinium is produced instead. Nitration of acetanilide gives only traces of 2-nitro isomer is obtained due to the great steric effect of the amide. Sulfonation is usually used to block the 4 position and increases the effectiveness to 56%.[3][4]
Uses and reactions
2-Nitroaniline is the main precursor to phenylenediamines, which are converted to benzimidazoles, a family of heterocycles that are key components in pharmaceuticals.[2]
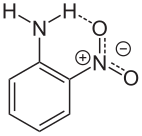
Aside from its reduction to phenylenediamine, 2-nitroaniline undergoes other reactions anticipated for aromatic amines. It is protonated to give the anilinium salts. Owing to the influence of the nitro substituent, the amine exhibits a basicity nearly 100,000x lower than aniline itself. Diazotization gives diazonium derivative,[5] which is a precursor to some diazo dyes. Acetylation affords 2-nitroacetanilide.
See also
References
- Safety data for o-nitroaniline
- Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
- T. W. Grahan, Solomons; Craig, B. Fryhle; Scott, A. Snyder (2011). Organic chemistry (11 ed.). pp. 606–607. ISBN 978-1119077251.
- Louis Ehrenfeld, Milton Puterbaugh (1929). "o-Nitrianiline". Organic Syntheses. 9: 64. doi:10.15227/orgsyn.009.0064.
- G. Wittig; R. W. Hoffmann (1967). "1,2,3-Benzothiadiazole 1,1-Dioxide". Org. Synth. 47: 4. doi:10.15227/orgsyn.047.0004.