PPIB
Peptidyl-prolyl cis-trans isomerase B is an enzyme that is encoded by the PPIB gene.[5] As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate protein folding of type I collagen.[6][7] Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved.
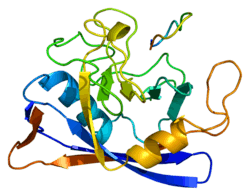
Structure
Like other cyclophilins, PPIB forms a β-barrel structure with a hydrophobic core. This β-barrel is composed of eight anti-parallel β-strands and capped by two α-helices at the top and bottom. In addition, the β-turns and loops in the strands contribute to the flexibility of the barrel.[8] In particular, PPIB is a 21 kDa protein which contains a C-terminal ER retention motif that directs the protein to the ER organelle, while its N-terminal extension attaches it to its substrates.[7][9]
Function
PPIB is a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family. PPIases catalyze the cis-trans isomerization of proline imidic peptide bonds and regulate protein folding and maturation. Proline is the only amino acid known to exist in both the cis and trans isomerization rate in vivo, and is often the rate-limiting step in protein refolding.[10] The PPIase family is further divided into three structurally distinct subfamilies: cyclophilin (CyP), FK506-binding protein (FKBP), and parvulin (Pvn).[11][12] While each family demonstrates PPIase activity, the families have no sequence of structural similarities. As a cyclophilin, PPIB binds cyclosporin A (CsA) and can be found within in the cell or secreted by the cell.[9][13]
Human PPIB
PPIB is the second of 18 cyclophilins to be identified in humans, after CypA.[11][13] PPIB localizes to the endoplasmic reticulum (ER) and participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer.[9][14] It is also associated with viral infections. In eukaryotes, cyclophilins localize ubiquitously to many cell and tissue types.[9][8] In addition to PPIase and protein chaperone activities, cyclophilins function in mitochondrial metabolism, apoptosis, immunological response, inflammation, and cell growth and proliferation.[6][9][8] Along with PPIC, PPIB localizes to the endoplasmic reticulum (ER), where it maintains redox homeostasis. Depletion of these two cyclophilins leads to hyperoxidation of the ER.[15]
In the ER, PPIB interacts with proteins such as P3H1, CRTAP, BiP, GRP94, PDI, and calreticulin to form foldase and chaperone complexes and facilitate protein folding, especially for type I collagen.[16][17] This protein is the major PPIase for type I collagen, since the collagen contains an abundance of prolines that require cis-trans isomerization for proper folding. Thus, PPIB is essential for collagen biosynthesis and post-translational modification and affects fibril assembly, matrix cross-linking, and bone mineralization.[16]
In addition, it is associated with the secretory pathway and released in biological fluids. This protein can bind to cells derived from T- and B-lymphocytes, and may regulate cyclosporine A-mediated immunosuppression.[18] In one experiment, the addition of PPIB into cell cultures in vitro induced chemotaxis and integrin-mediated adhesion of T cells to the extracellular matrix (ECM), suggesting that it might function in innate immunity by recruiting T cells into infected tissue in vivo.[9]
Clinical significance
As a cyclophilin, PPIB binds the immunosuppressive drug CsA to form a CsA-cyclophilin complex, which then targets calcineurin to inhibit the signaling pathway for T-cell activation.
In cardiac myogenic cells, cyclophilins have been observed to be activated by heat shock and hypoxia-reoxygenation as well as complex with heat shock proteins. Thus, cyclophilins may function in cardioprotection during ischemia-reperfusion injury.[9]
PPIB contributes to the replication and infection of viruses causing diseases such as AIDS, hepatitis C, measles, and influenza A. Thus, therapeutic targeting of PPIB with selective inhibitors may prove effective in combating viral infections and inflammation.[7] Currently, PPIB is employed as a biomarker for various types of cancer.[14] Moreover, there are two antigenic epitopes (CypB84-92 and CypB91-99) recognized by HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes which could be used as cancer vaccines, and in fact, were used to treat lung cancer in a clinical trial.[9]
Bacterial PPIB
PPIB has been identified in both Gram-negative bacteria and Gram-positive bacteria as an intracellular protein. In Escherichia coli, PPIB has been shown to have both PPIase activity and Chaperone (protein) activity.[19] In Staphylococcus aureus, PPIB has been shown to have PPIase activity, and to directly assist in the refolding of Staphylococcal nuclease.[20] Aside from these bacteria, PPIB has been identified in Brucella abortus, Mycobacterium tuberculosis, Bacillus subtilis and other bacteria.[21][22][23]
Interactions
PPIB has been shown to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000166794 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000032383 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Price ER, Zydowsky LD, Jin MJ, Baker CH, McKeon FD, Walsh CT (Apr 1991). "Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence". Proc Natl Acad Sci U S A. 88 (5): 1903–7. Bibcode:1991PNAS...88.1903P. doi:10.1073/pnas.88.5.1903. PMC 51134. PMID 2000394.
- Kazui T, Inoue N, Yamada O, Komatsu S (Jan 1992). "Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment". The Annals of Thoracic Surgery. 53 (1): 109–14. doi:10.1016/0003-4975(92)90767-x. PMID 1530810.
- Hoffmann H, Schiene-Fischer C (Jul 2014). "Functional aspects of extracellular cyclophilins". Biological Chemistry. 395 (7–8): 721–35. doi:10.1515/hsz-2014-0125. PMID 24713575.
- Wang T, Yun CH, Gu SY, Chang WR, Liang DC (Aug 2005). "1.88 A crystal structure of the C domain of hCyP33: a novel domain of peptidyl-prolyl cis-trans isomerase". Biochemical and Biophysical Research Communications. 333 (3): 845–9. doi:10.1016/j.bbrc.2005.06.006. PMID 15963461.
- Yao Q, Li M, Yang H, Chai H, Fisher W, Chen C (Mar 2005). "Roles of cyclophilins in cancers and other organ systems". World Journal of Surgery. 29 (3): 276–80. doi:10.1007/s00268-004-7812-7. PMID 15706440.
- Göthel, S. F.; Marahiel, M. A. (March 1999). "Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts". Cellular and Molecular Life Sciences. 55 (3): 423–436. doi:10.1007/s000180050299. ISSN 1420-682X. PMID 10228556.
- Kazui T, Inoue N, Yamada O, Komatsu S (Jan 1992). "Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment". The Annals of Thoracic Surgery. 53 (1): 109–14. doi:10.1016/0003-4975(92)90767-x. PMID 1530810.
- Wang T, Yun CH, Gu SY, Chang WR, Liang DC (Aug 2005). "1.88 A crystal structure of the C domain of hCyP33: a novel domain of peptidyl-prolyl cis-trans isomerase". Biochemical and Biophysical Research Communications. 333 (3): 845–9. doi:10.1016/j.bbrc.2005.06.006. PMID 15963461.
- Hoffmann H, Schiene-Fischer C (Jul 2014). "Functional aspects of extracellular cyclophilins". Biological Chemistry. 395 (7–8): 721–35. doi:10.1515/hsz-2014-0125. PMID 24713575.
- Ray P, Rialon-Guevara KL, Veras E, Sullenger BA, White RR (May 2012). "Comparing human pancreatic cell secretomes by in vitro aptamer selection identifies cyclophilin B as a candidate pancreatic cancer biomarker". The Journal of Clinical Investigation. 122 (5): 1734–41. doi:10.1172/JCI62385. PMC 3336995. PMID 22484812.
- Stocki P, Chapman DC, Beach LA, Williams DB (Aug 2014). "Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum redox homeostasis". The Journal of Biological Chemistry. 289 (33): 23086–96. doi:10.1074/jbc.M114.570911. PMC 4132807. PMID 24990953.
- Cabral WA, Perdivara I, Weis M, Terajima M, Blissett AR, Chang W, Perosky JE, Makareeva EN, Mertz EL, Leikin S, Tomer KB, Kozloff KM, Eyre DR, Yamauchi M, Marini JC (Jun 2014). "Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta". PLoS Genetics. 10 (6): e1004465. doi:10.1371/journal.pgen.1004465. PMC 4072593. PMID 24968150.
- Ishikawa Y, Bächinger HP (Nov 2013). "An additional function of the rough endoplasmic reticulum protein complex prolyl 3-hydroxylase 1·cartilage-associated protein·cyclophilin B: the CXXXC motif reveals disulfide isomerase activity in vitro". The Journal of Biological Chemistry. 288 (44): 31437–46. doi:10.1074/jbc.M113.498063. PMC 3814740. PMID 24043621.
- "Entrez Gene: PPIB peptidylprolyl isomerase B (cyclophilin B)".
- Skagia, Aggeliki; Vezyri, Eleni; Sigala, Markezina; Kokkinou, Areti; Karpusas, Michael; Venieraki, Anastasia; Katinakis, Panagiotis; Dimou, Maria (January 2017). "Structural and functional analysis of cyclophilin PpiB mutants supports an in vivo function not limited to prolyl isomerization activity". Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 22 (1): 32–44. doi:10.1111/gtc.12452. ISSN 1365-2443. PMID 27868330.
- Wiemels, Richard E.; Cech, Stephanie M.; Meyer, Nikki M.; Burke, Caleb A.; Weiss, Andy; Parks, Anastacia R.; Shaw, Lindsey N.; Carroll, Ronan K. (2017-01-01). "An Intracellular Peptidyl-Prolyl cis/trans Isomerase Is Required for Folding and Activity of the Staphylococcus aureus Secreted Virulence Factor Nuclease". Journal of Bacteriology. 199 (1). doi:10.1128/JB.00453-16. ISSN 1098-5530. PMC 5165095. PMID 27795319.
- Roset, Mara S.; García Fernández, Lucía; DelVecchio, Vito G.; Briones, Gabriel (February 2013). "Intracellularly Induced Cyclophilins Play an Important Role in Stress Adaptation and Virulence of Brucella abortus". Infection and Immunity. 81 (2): 521–530. doi:10.1128/IAI.01125-12. ISSN 0019-9567. PMC 3553818. PMID 23230297.
- Göthel, S. F.; Scholz, C.; Schmid, F. X.; Marahiel, M. A. (1998-09-22). "Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions". Biochemistry. 37 (38): 13392–13399. doi:10.1021/bi981253w. ISSN 0006-2960. PMID 9748346.
- Pandey, Saurabh; Sharma, Ashish; Tripathi, Deeksha; Kumar, Ashutosh; Khubaib, Mohd; Bhuwan, Manish; Chaudhuri, Tapan Kumar; Hasnain, Seyed Ehtesham; Ehtesham, Nasreen Zafar (2016-03-16). "Mycobacterium tuberculosis Peptidyl-Prolyl Isomerases Also Exhibit Chaperone like Activity In-Vitro and In-Vivo". PLoS ONE. 11 (3): e0150288. Bibcode:2016PLoSO..1150288P. doi:10.1371/journal.pone.0150288. ISSN 1932-6203. PMC 4794191. PMID 26981873.
- Zhang J, Herscovitz H (Feb 2003). "Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B". J. Biol. Chem. 278 (9): 7459–68. doi:10.1074/jbc.M207976200. PMID 12397072.
Further reading
- Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (1993). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes". Electrophoresis. 13 (12): 960–9. doi:10.1002/elps.11501301199. PMID 1286667.
- Peddada LB, McPherson JD, Law R, Wasmuth JJ, Youderian P, Deans RJ (1992). "Somatic cell mapping of the human cyclophilin B gene (PPIB) to chromosome 15". Cytogenet. Cell Genet. 60 (3–4): 219–21. doi:10.1159/000133343. PMID 1505219.
- Arber S, Krause KH, Caroni P (1992). "s-cyclophilin is retained intracellularly via a unique COOH-terminal sequence and colocalizes with the calcium storage protein calreticulin". J. Cell Biol. 116 (1): 113–25. doi:10.1083/jcb.116.1.113. PMC 2289259. PMID 1530944.
- Hasel KW, Glass JR, Godbout M, Sutcliffe JG (1991). "An endoplasmic reticulum-specific cyclophilin". Mol. Cell. Biol. 11 (7): 3484–91. doi:10.1128/mcb.11.7.3484. PMC 361082. PMID 1710767.
- Spik G, Haendler B, Delmas O, Mariller C, Chamoux M, Maes P, Tartar A, Montreuil J, Stedman K, Kocher HP (1991). "A novel secreted cyclophilin-like protein (SCYLP)". J. Biol. Chem. 266 (17): 10735–8. PMID 2040592.
- Bram RJ, Crabtree GR (1994). "Calcium signalling in T cells stimulated by a cyclophilin B-binding protein". Nature. 371 (6495): 355–8. Bibcode:1994Natur.371..355B. doi:10.1038/371355a0. PMID 7522304.
- Allain F, Boutillon C, Mariller C, Spik G (1995). "Selective assay for CyPA and CyPB in human blood using highly specific anti-peptide antibodies". J. Immunol. Methods. 178 (1): 113–20. doi:10.1016/0022-1759(94)00249-V. PMID 7829860.
- Price ER, Jin M, Lim D, Pati S, Walsh CT, McKeon FD (1994). "Cyclophilin B trafficking through the secretory pathway is altered by binding of cyclosporin A". Proc. Natl. Acad. Sci. U.S.A. 91 (9): 3931–5. Bibcode:1994PNAS...91.3931P. doi:10.1073/pnas.91.9.3931. PMC 43696. PMID 7909608.
- Mikol V, Kallen J, Walkinshaw MD (1994). "X-ray structure of a cyclophilin B/cyclosporin complex: comparison with cyclophilin A and delineation of its calcineurin-binding domain". Proc. Natl. Acad. Sci. U.S.A. 91 (11): 5183–6. Bibcode:1994PNAS...91.5183M. doi:10.1073/pnas.91.11.5183. PMC 43956. PMID 8197205.
- Allain F, Denys A, Spik G (1994). "Characterization of surface binding sites for cyclophilin B on a human tumor T-cell line". J. Biol. Chem. 269 (24): 16537–40. PMID 8206968.
- Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP (1993). "Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B". Cell. 73 (6): 1067–78. doi:10.1016/0092-8674(93)90637-6. PMID 8513493.
- Braaten D, Ansari H, Luban J (1997). "The hydrophobic pocket of cyclophilin is the binding site for the human immunodeficiency virus type 1 Gag polyprotein". J. Virol. 71 (3): 2107–13. PMC 191305. PMID 9032343.
- Montague JW, Hughes FM, Cidlowski JA (1997). "Native recombinant cyclophilins A, B, and C degrade DNA independently of peptidylprolyl cis-trans-isomerase activity. Potential roles of cyclophilins in apoptosis". J. Biol. Chem. 272 (10): 6677–84. doi:10.1074/jbc.272.10.6677. PMID 9045699.
- Denys A, Allain F, Foxwell B, Spik G (1997). "Distribution of cyclophilin B-binding sites in the subsets of human peripheral blood lymphocytes". Immunology. 91 (4): 609–17. doi:10.1046/j.1365-2567.1997.00296.x. PMC 1363883. PMID 9378502.
- Endrich MM, Gehring H (1998). "The V3 loop of human immunodeficiency virus type-1 envelope protein is a high-affinity ligand for immunophilins present in human blood". Eur. J. Biochem. 252 (3): 441–6. doi:10.1046/j.1432-1327.1998.2520441.x. PMID 9546659.
- Endrich MM, Gehrig P, Gehring H (1999). "Maturation-induced conformational changes of HIV-1 capsid protein and identification of two high affinity sites for cyclophilins in the C-terminal domain". J. Biol. Chem. 274 (9): 5326–32. doi:10.1074/jbc.274.9.5326. PMID 10026140.
- Bristow R, Byrne J, Squirell J, Trencher H, Carter T, Rodgers B, Saman E, Duncan J (1999). "Human cyclophilin has a significantly higher affinity for HIV-1 recombinant p55 than p24". J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20 (4): 334–6. doi:10.1097/00042560-199904010-00002. PMID 10096576.
- Rycyzyn MA, Reilly SC, O'Malley K, Clevenger CV (2001). "Role of cyclophilin B in prolactin signal transduction and nuclear retrotranslocation". Mol. Endocrinol. 14 (8): 1175–86. doi:10.1210/me.14.8.1175. PMID 10935542.
- Yurchenko V, O'Connor M, Dai WW, Guo H, Toole B, Sherry B, Bukrinsky M (2001). "CD147 is a signaling receptor for cyclophilin B". Biochem. Biophys. Res. Commun. 288 (4): 786–8. doi:10.1006/bbrc.2001.5847. PMID 11688976.