1,4-Benzoquinone
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone.[4] The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexa-2,5-diene-1,4-dione[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
| 773967 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.097 | ||
| EC Number |
| ||
| 2741 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2587 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H4O2 | |||
| Molar mass | 108.096 g·mol−1 | ||
| Appearance | Yellow solid | ||
| Odor | Acrid, chlorine-like[2] | ||
| Density | 1.318 g/cm3 at 20 °C | ||
| Melting point | 115 °C (239 °F; 388 K) | ||
| Boiling point | Sublimes | ||
| 11 g/L (18 °C) | |||
| Solubility | Slightly soluble in petroleum ether; soluble in acetone; 10% in ethanol, benzene, diethyl ether | ||
| Vapor pressure | 0.1 mmHg (25°C)[2] | ||
| -38.4·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Toxic | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H301, H315, H319, H331, H335, H400 | ||
| P261, P264, P270, P271, P273, P280, P301+310, P302+352, P304+340, P305+351+338, P311, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |||
| Flash point | 38 to 93 °C; 100 to 200 °F; 311 to 366 K[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
296 mg/kg (mammal, subcutaneous) 93.8 mg/kg (mouse, subcutaneous) 8.5 mg/kg (mouse, IP) 5.6 mg/kg (rat) 130 mg/kg (rat, oral) 25 mg/kg (rat, IV)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.4 mg/m3 (0.1 ppm)[2] | ||
REL (Recommended) |
TWA 0.4 mg/m3 (0.1 ppm)[2] | ||
IDLH (Immediate danger) |
100 mg/m3[2] | ||
| Related compounds | |||
Related compounds |
1,2-Benzoquinone | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
1,4-Benzoquinone is prepared industrially by oxidation of hydroquinone, which can be obtained by several routes. One route involves oxidation of diisopropylbenzene and the Hock rearrangement. The net reaction can be represented as follows:
- C6H4(CHMe2)2 + 3 O2 → C6H4O2 + 2 OCMe2 + H2O
The reaction proceeds via the bis(hydroperoxide) and the hydroquinone. Acetone is a coproduct.[5]
Another major process involves the direct hydroxylation of phenol by acidic hydrogen peroxide: C6H5OH + H2O2 → C6H4(OH)2 + H2O Both hydroquinone and catechol are produced. Subsequent oxidation of the hydroquinone gives the quinone.[6]
Quinone was originally prepared industrially by oxidation of aniline, for example by manganese dioxide.[7] This method is mainly practiced in PRC where environmental regulations are more relaxed.
Oxidation of hydroquinone is facile.[4][8] One such method makes use of hydrogen peroxide as the oxidizer and iodine or an iodine salt as a catalyst for the oxidation occurring in a polar solvent; e.g. isopropyl alcohol.[9]
When heated to near its melting point, 1,4-benzoquinone sublimes, even at atmospheric pressure, allowing for an effective purification. Impure samples are often dark-colored due to the presence of quinhydrone, a dark green 1:1 charge-transfer complex of quinone with hydroquinone.[10]
Structure and redox
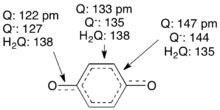
Benzoquinone is a planar molecule with localized, alternating C=C, C=O, and C–C bonds. Reduction gives the semiquinone anion C6H4O2−}, which adopts a more delocalized structure. Further reduction coupled to protonation gives the hydroquinone, wherein the C6 ring is fully delocalized.[11]
Reactions and applications
Quinone is mainly used as a precursor to hydroquinone, which is used in photography and rubber manufacture as a reducing agent and antioxidant.[6] Benzoquinonium is a Skeletal muscle relaxant, ganglion blocking agent that is made from benzoquinone.[12]
Organic synthesis
It is used as a hydrogen acceptor and oxidant in organic synthesis.[13] 1,4-Benzoquinone serves as a dehydrogenation reagent. It is also used as a dienophile in Diels Alder reactions.[14]
Benzoquinone reacts with acetic anhydride and sulfuric acid to give the triacetate of hydroxyquinol.[15][16] This reaction is called the Thiele reaction or Thiele–Winter reaction[17][18] after Johannes Thiele, who first described it in 1898, and after Ernst Winter, who further described its reaction mechanism in 1900. An application is found in this step of the total synthesis of Metachromin A:[19]
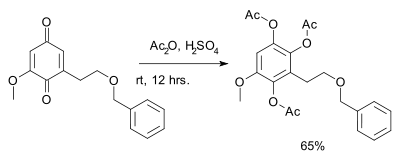 An application of the Thiele reaction, involving a benzoquinone derivative.
An application of the Thiele reaction, involving a benzoquinone derivative.
Benzoquinone is also used to suppress double-bond migration during olefin metathesis reactions.
An acidic potassium iodide solution reduces a solution of benzoquinone to hydroquinone, which can be reoxidized back to the quinone with a solution of silver nitrate.
Due to its ability to function as an oxidizer, 1,4-benzoquinone can be found in methods using the Wacker-Tsuji oxidation, wherein a palladium salt catalyzes the conversion of an alkene to a ketone. This reaction is typically carried out using pressurized oxygen as the oxidizer, but benzoquinone can sometimes preferred. It is also used as a reagent in some variants on Wacker oxidations.
1,4-Benzoquinone is used in the synthesis of Bromadol and related analogs.
Metabolism
1,4-Benzoquinone is a toxic metabolite found in human blood and can be used to track exposure to benzene or mixtures containing benzene and benzene compounds, such as petrol.[21] The compound can interfere with cellular respiration, and kidney damage has been found in animals receiving severe exposure. It is excreted in its original form and also as variations of its own metabolite, hydroquinone.[7]
Benzoquinone compounds are a metabolite of paracetamol.[22]
Safety

1,4-Benzoquinone is able to stain skin dark brown, cause erythema (redness, rashes on skin) and lead on to localized tissue necrosis. It is particularly irritating to the eyes and respiratory system. Its ability to sublime at commonly encountered temperatures allows for a greater airborne exposure risk than might be expected for a room-temperature solid. IARC has found insufficient evidence to comment on the compound's carcinogenicity, but has noted that it can easily pass into the bloodstream and that it showed activity in depressing bone marrow production in mice and can inhibit protease enzymes involved in cellular apoptosis.[7]
1,4-Benzoquinone is a constituent of tobacco smoke.[23]
Related 1,4-benzoquinones
A variety of derivatives and analogues are known. Ubiquinone-1 is a naturally occurring 1,4-benzoquinone that is involved in respiration apparatus. The benzoquinone blattellaquinone is a sex pheromone in cockroaches.
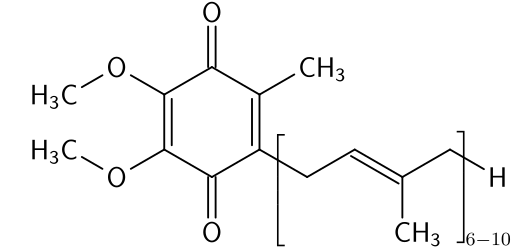 Ubiquinones, as their name implies, are ubiquitous in living creatures, being components of respiratory apparatus.
Ubiquinones, as their name implies, are ubiquitous in living creatures, being components of respiratory apparatus. Blattellaquinone, a sex pheromone in cockroaches.
Blattellaquinone, a sex pheromone in cockroaches.
Illustrative examples of quinones and derivatives of benzoquinone that are useful in industry and organic chemistry include:
- 1,4-Naphthoquinone, derived by oxidation of naphthalene with chromium trioxide.[24]
- 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), a stronger oxidant and dehydrogenation agent than 1,4-benzoquinone.[25]
- Chloro-p-benzoquinone, (CAS no. [695-99-8])[26]
- Chloranil, 1,4-C6Cl4O2, a stronger oxidant and dehydrogenation agent than 1,4-benzoquinone.
- Ambazone can be obtained in a two step synthetic method, reacting 1,4-benzoquinone with aminoguanidine and thiosemicarbazide.
- Mecarbinate (dimecarbine) is made by the reaction of ethyl N-methyl-β-aminocrotonate with para-benzoquinone.
- Amendol
- Oxyphemedol
- Phemedol all in FR5142 (M) ― 1967-06-05. Note: These are all indoles made via the Nenitzescu indole synthesis.
- Apaziquone is antineoplastic.
 A few grams of para-benzoquinone, prepared by the action of iodine and hydrogen peroxide on hydroquinone, after being sublimed in a 3-litre beaker
A few grams of para-benzoquinone, prepared by the action of iodine and hydrogen peroxide on hydroquinone, after being sublimed in a 3-litre beaker A 200mm watch glass covered in freshly sublimated para-benzoquinone
A 200mm watch glass covered in freshly sublimated para-benzoquinone A sample of quinhydrone
A sample of quinhydrone
See also
- Tetrahydroxybenzoquinone
- Benzoquinonetetracarboxylic acid
- 1,2-Benzoquinone
- Quinones
- Duroquinone
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 723–724. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0542". National Institute for Occupational Safety and Health (NIOSH).
- "Quinone". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Underwood, H. W. Jr.; Walsh, W. L. (1936). "Quinone". Organic Syntheses. 16: 73. doi:10.15227/orgsyn.002.0085.; Collective Volume, 2, p. 553
- Gerhard Franz, Roger A. Sheldon "Oxidation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 doi:10.1002/14356007.a18_261
- Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_499.
- "1,4-Benzoquinone (para-Quinone)" (PDF). IARC Monograph.
- Vliet, E. B. (1922). "Quinone". Organic Syntheses. 2: 85. doi:10.15227/orgsyn.016.0073.; Collective Volume, 1, p. 482
- US patent 4973720, "Process for the preparation of p-benzoquinone"
- Sakurai, T. (1968). "On the refinement of the crystal structures of phenoquinone and monoclinic quinhydrone". Acta Crystallographica Section B. 24 (3): 403–412. doi:10.1107/S0567740868002451.
- Lü, Jian-Ming; Rosokha, Sergiy V; Neretin, Ivan S; Kochi, Jay K (2006). "Quinones as Electron Acceptors. X-Ray Structures, Spectral (EPR, UV−vis) Characteristics and Electron-Transfer Reactivities of Their Reduced Anion Radicals as Separated vs Contact Ion Pairs". Journal of the American Chemical Society. 128 (51): 16708–19. doi:10.1021/ja066471o. PMID 17177421.
- Cavallito, Chester J.; Soria, Albert E.; Hoppe, James O. (1950). "Amino- and Ammonium-alkylaminobenzoquinones as Curarimimetic Agents". Journal of the American Chemical Society. 72 (6): 2661–2665. doi:10.1021/ja01162a088. ISSN 0002-7863.
- Yang, T.-K.; Shen, C.-Y. (2004). "1,4-Benzoquinone". In L. Paquette (ed.). Encyclopedia of Reagents for Organic Synthesis. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rb033. ISBN 978-0471936237.
- Oda, M.; Kawase, T.; Okada, T.; Enomoto, T. (1996). "2-Cyclohexene-1,4-dione". Organic Syntheses. 73: 253. doi:10.15227/orgsyn.073.0253.; Collective Volume, 9, p. 186
- Vliet, E. B. (1941). "Hydroquinone Triacetate". Organic Syntheses. 1: 317. doi:10.15227/orgsyn.004.0035.
- Knowles, M. B. (1952). "Process for production of 2,4,5-trihydroxyacetophenone" (PDF). Google Patents. Eastman Kodak Co. Retrieved 24 December 2014.
- McOmie, J. F. W.; Blatchly, J. M. (2011). "The Thiele-Winter Acetoxylation of Quinones". Organic Reactions. 19: 199–277. doi:10.1002/0471264180.or019.03. ISBN 9780471196198.
- Thiele, J. (1898). "Ueber die Einwirkung von Essigsäure-anhydrid auf Chinon und auf Dibenzoylstyrol". Berichte der Deutschen Chemischen Gesellschaft. 31 (1): 1247–1249. doi:10.1002/cber.189803101226.
- Almeida, W. P.; Correia, C. R. D. (1999). "Stereoselective Total Synthesis and Enantioselective Formal Synthesis of the Antineoplastic Sesquiterpene Quinone Metachromin A" (PDF). Journal of the Brazilian Chemical Society. 10 (5): 401–414. doi:10.1590/S0103-50531999000500011.
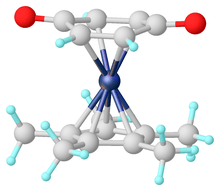
- Moussa, Jamal; Guyard-Duhayon, Carine; Herson, Patrick; Amouri, Hani; Rager, Marie Noelle; Jutand, Anny (2004). "η5-Semiquinone Complexes and the Related η4-Benzoquinone of (Pentamethylcyclopentadienyl)rhodium and -iridium: Synthesis, Structures, Hydrogen Bonding, and Electrochemical Behavior". Organometallics. 23: 6231–6238. doi:10.1021/om049292t.
- Lin, Y. S.; McKelvey, W.; Waidyanatha, S.; Rappaport, S. M. (2006). "Variability of Albumin Adducts of 1,4-Benzoquinone, a Toxic Metabolite of Benzene, in Human Volunteers". Biomarkers. 11 (1): 14–27. doi:10.1080/13547500500382975. PMID 16484134.
- Dahlin, D. C.; Miwa, G. T.; Lu, A. Y.; Nelson, S. D. (1984). "N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen". Proceedings of the National Academy of Sciences of the United States of America. 81 (5): 1327–1331. Bibcode:1984PNAS...81.1327D. doi:10.1073/pnas.81.5.1327. PMC 344826. PMID 6424115.
- Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
- Braude E. A.; Fawcett, J. S. (1953). "1,4-Naphthoquinone". Organic Syntheses. 33: 50. doi:10.15227/orgsyn.033.0050.; Collective Volume, 4, p. 698
- Vogel, E.; Klug, W.; Breuer, A. (1974). "1,6-Methano[10]annulene". Organic Syntheses. 54: 11. doi:10.15227/orgsyn.054.0011.; Collective Volume, 6, p. 731
- Harman, R. E. (1955). "Chloro-p-benzoquinone". Organic Syntheses. 35: 22. doi:10.15227/orgsyn.035.0022.; Collective Volume, 4, p. 148