Oxanorbornadiene
Oxanorbornadiene (OND) is a bicyclic organic compound with an oxygen atom bridging the two opposing saturated carbons of 1,4-cyclohexadiene. OND is related to all-carbon bicycle norbornadiene.
 | |
| Names | |
|---|---|
| IUPAC name
7-oxabicyclo[2.2.1]hepta-2,5-diene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6O | |
| Molar mass | 94.11 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis

While unsubstituted OND is known, the most useful OND derivatives are dialkyl OND-2,3-dicarboxylates, readily obtainable by a Diels–Alder cycloaddition between furans and acetylenedicarboxylates such as DMAD.[1]
Properties
OND-2,3-dicarboxylates (thereafter referred to as OND) display unusually high reactivity towards azides[2] and thiols.[3] OND–thiol adducts are prone to retro-Diels–Alder fragmentation, which occurs with widely variable rates.[4]
Reactions with Azides
ONDs react with azides to yield a mixture of four products, arising from initial 1,3-dipolar cycloaddition onto either of the two olefins, followed by retro-Diels–Alder cycloreversion to form 1,2,3-triazoles and furans. The intermediate triazolines avoid detection because of a very strong thermodynamic drive to collapse into two aromatic products. The relative preference of attack on either double bond is governed by the electronic nature of the azides. Electron-rich aliphatic azides, e.g. benzyl azide, react preferentially via their HOMO orbital. Interaction of the azide HOMO with LUMO orbital of the OND, localized on the electron-poor C-2 and C-3, leads the products consistent with path A. Electron-poor aryl azides, such as p-nitrophenyl azide, react, to a significant extent, via their LUMO orbitals, interacting with OND HOMO (C-5 and C-6), leading to higher amounts of path B products. The dipolar reactivity of OND with azides is unusually high for olefins, and even exceeds that of parent electron-poor alkyne DMAD.[2][5]
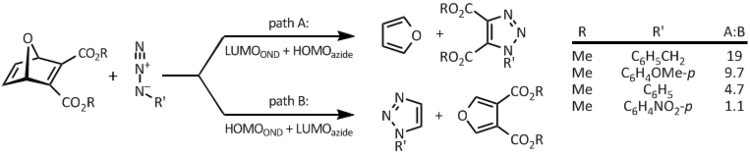
References
- Diels, Otto; Alder, Kurt (1931). "Synthesen in der hydroaromatischen Reihe. XII. Mitteilung. („Dien-Synthesen" sauerstoffhaltiger Heteroringe. 2. Dien-Synthesen des Furans.)". Annalen. 490 (1): 243–257. doi:10.1002/jlac.19314900110.
- Van Berkel, Sander S.; Dirks, A. (Ton) J.; Debets, Marjoke F.; Van Delft, Floris L.; Cornelissen, Jeroen J. L. M.; Nolte, Roeland J. M.; Rutjes, Floris P. J. T. (2007). "Metal-Free Triazole Formation as a Tool for Bioconjugation". ChemBioChem. 8 (13): 1504–8. doi:10.1002/cbic.200700278. PMID 17631666.
- Hong, Vu P.; Kislukhin, Alexander A.; Finn, M.G. (2009). "Thiol-Selective Fluorogenic Probes for Labeling and Release". J. Am. Chem. Soc. 131 (29): 9986–94. doi:10.1021/ja809345d. PMC 3166955. PMID 19621956.
- Kislukhin, Alexander A.; Higginson, Cody J.; Hong, Vu P.; Finn, M.G. (2012). "Degradable Conjugates from Oxanorbornadiene Reagents". J. Am. Chem. Soc. 134 (14): 6491–7. doi:10.1021/ja301491h. PMC 3432588. PMID 22455380.
- Cristina, D.; De Amici, M.; De Micheli, S.; Gandolfi, R. (1981). "Site selectivity in the reactions of 1,3-dipoles with norbornadiene derivatives". Tetrahedron. 37: 1349–57. doi:10.1016/S0040-4020(01)92451-2.