Organotantalum chemistry
Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
Classes of organotantalum compounds
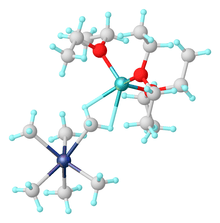
Alkyl and aryl complexes
Pentamethyltantalum was reported by Richard Schrock in 1974.[1]
Salts of [Ta(CH3)6]− are prepared by alkylation of TaF5 using methyl lithium:[2]
- TaF5 + 6 LiCH3 → Li[Ta(CH3)6] + 5 LiF
Alkylidene complexes
Tantalum alkylidene complexes arise by treating trialkyltantalum dichloride with alkyl lithium reagents. This reaction initially forms a thermally unstable tetraalkyl-monochloro-tantalum complex, which undergoes α-hydrogen elimination, followed by alkylation of the remaining chloride.[1]
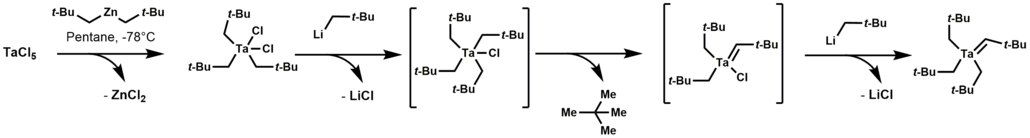
Tantalum alkylidene complexes are nucleophilic.[1] They effect a number of reactions including: olefinations, olefin metathesis, hydroaminoalkylation of olefins, and conjugate allylation of enones.
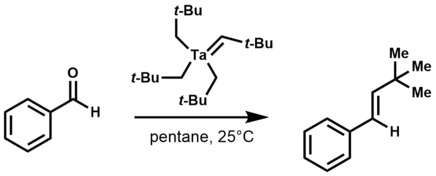
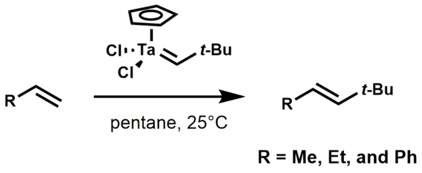
Ethylene, propylene, and styrene react with tantalum alkylidene complexes to yield olefin metathesis products.[3]
Cyclopentadienyl complexes
Some of the first reported organotantalum complexes were cyclopentadienyl derivatives. These arise from the salt metathesis reactions of sodium cyclopentadienide and tantalum pentachloride. More soluble and better developed are derivatives of pentamethylcyclopentadiene such as Cp*TaCl4, Cp*2TaCl2, and Cp*2TaH3.[4]
Tantalum carbonyls
Reduction of TaCl5 under an atmosphere of CO gives the salts of [Ta(CO)6]−.[5] These same anions can be obtained by carbonylation of tantalum arene complex]]es.
Tantalum arenes and alkyne complexes
Treatment of tantalum pentachloride with hexamethylbenzene (C6Me6), aluminium, and aluminium trichloride gives M(η6-C6Me6)AlCl4]2.[6]
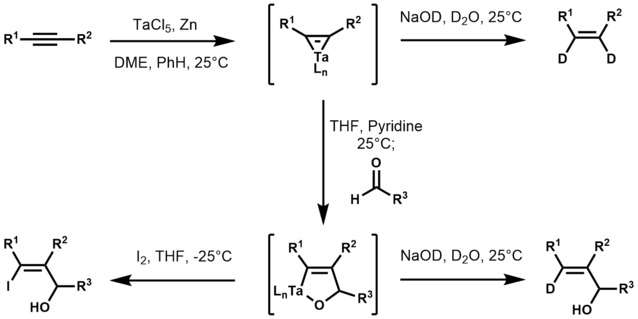
Uncharacterized tantalum-alkyne complexes were described in the 1970s.[7] Some catalyze cyclotrimerizations.[8][9] The synthetic utility of tantalum-alkyne complexes was later expanded by K. Utimoto in 1989 when he used tantalum-alkyne complexes to synthesize trisubstituted allylic alcohols from acetylenes and aldehydes.[10] In this work, Utimoto first reduces tantalum(V) chloride with zinc to produce a low-valent tantalum species, which readily reacts with acetylenes to yield a tantalum-cyclopropene. Treatment of the tantalum-cyclopropene with THF, pyridine, and an aldehyde results in the formation of an oxatantala-cyclopentene, which upon aqueous work-up affords (E)-allylic alcohols exclusively. Utimoto also noted that treatment of the oxatantala-cyclopentene with iodine resulted in the corresponding iodo alcohol.
Applications
Organotantalum compounds are of academic interest, but few or no commercial applications have been described.
Tantalum-amido complexes
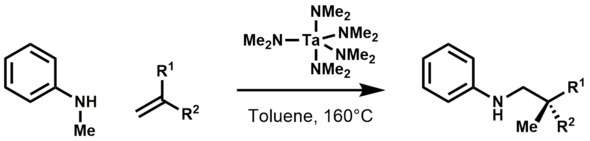
Organotantalum compounds are invoked as intermediates in C-alkylation of secondary amines with 1-alkenes using Ta(NMe2)5.[11] The chemistry developed by Maspero was later brought to fruition when Hartwig and Herzon reported the hydroaminoalkylation of olefins to form alkylamines:[12]
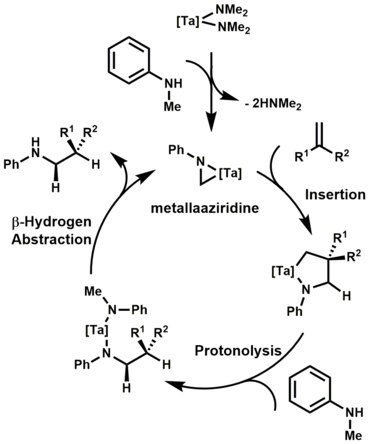
Mechanistically, the first step of the catalytic cycle is believed to be the C-H activation (via β-hydrogen abstraction) of the bisamide, which results in the formation of a metallaaziridine. Subsequent olefin insertion, protonolysis of the tantalum-carbon bond, and β-hydrogen abstraction affords the alkylamine product.[13] Notable advancements in this area were also made by Doye[14] and Schafer[15] when they showed tantalum catalyzed hydroaminoalkylations were exclusively branch selective and highly diastereoselective.
Transmetalation
Organotantalum reagents arise via transmetalation of organotin compounds with tantalum(V) chloride.[16] These organotantalum reagents promote the conjugate allylation of enones. Although the direct allylation of carbonyl groups is prevalent throughout the literature, little has been reported on the conjugate allylation of enones.[17]
References
- Schrock, Richard R. (1979-03-01). "Alkylidene complexes of niobium and tantalum". Accounts of Chemical Research. 12 (3): 98–104. doi:10.1021/ar50135a004. ISSN 0001-4842.
- Kleinhenz, S.; Pfennig, V.; Seppelt, K. (1998). "Preparation and Structures of [W(CH3)6], [Re(CH3)6], [Nb(CH3)6]−, and [Ta(CH3)6]−". Chem. Eur. J. 4 (9): 1687. doi:10.1002/(SICI)1521-3765(19980904)4:9<1687::AID-CHEM1687>3.0.CO;2-R.
- McLain, S. J.; Wood, C. D.; Schrock, R. R. (1977-05-01). "Multiple metal-carbon bonds. 6. The reaction of niobium and tantalum neopentylidene complexes with simple olefins: a route to metallocyclopentanes". Journal of the American Chemical Society. 99 (10): 3519–3520. doi:10.1021/ja00452a064. ISSN 0002-7863.
- Endy Y.-J. Min, John E. Bercaw (2014). Bis(η5-Pentamethylcyclopentadienyl) Complexes of Niobium and Tantalum. Inorg. Synth. Inorganic Syntheses. 36. pp. 52–57. doi:10.1002/9781118744994.ch11. ISBN 9781118744994.CS1 maint: uses authors parameter (link)
- J. E. Ellis, A. Davison (1976). Tris[Bis(2-Methoxyethyl)Ether]Potassium and Tetraphenylarsonium Hexacarbonylmetallates(1–) of Niobium and Tantalum. Inorg. Synth. Inorganic Syntheses. 16. pp. 68–73. doi:10.1002/9780470132470.ch21. ISBN 9780470132470.CS1 maint: uses authors parameter (link)
- Pampaloni, G. (2010). "Aromatic hydrocarbons as ligands. Recent advances in the synthesis, the reactivity and the applications of bis(η6-arene) complexes". Coordination Chemistry Reviews. 254 (5–6): 402–419. doi:10.1016/j.ccr.2009.05.014.CS1 maint: uses authors parameter (link)
- Labinger, Jay A.; Schwartz, Jeffrey; Townsend, John M. (1974-06-01). "Iodo- and hydridotantalum(III) complexes of dialkylacetylenes". Journal of the American Chemical Society. 96 (12): 4009–4011. doi:10.1021/ja00819a047. ISSN 0002-7863.
- Cotton, F. Albert; Hall, William T. (1979-08-01). "Reactions of tantalum(III) with alkynes and nitriles". Journal of the American Chemical Society. 101 (17): 5094–5095. doi:10.1021/ja00511a064. ISSN 0002-7863.
- Bruck, M. A.; Copenhaver, A. S.; Wigley, D. E. (1987-10-01). "Alkyne cyclizations at reduced tantalum centers: synthesis and molecular structure of (.eta.6-C6Me6)Ta(O-2,6-i-Pr2C6H3)2Cl". Journal of the American Chemical Society. 109 (21): 6525–6527. doi:10.1021/ja00255a056. ISSN 0002-7863.
- Takai, Kazuhiko; Kataoka, Y.; Utimoto, K. (1990-03-01). "Tantalum-alkyne complexes as synthetic intermediates. Stereoselective preparation of trisubstituted allylic alcohols from acetylenes and aldehydes". The Journal of Organic Chemistry. 55 (6): 1707–1708. doi:10.1021/jo00293a008. ISSN 0022-3263.
- Clerici, Mario G.; Maspero, Federico (1980-01-01). "Catalytic C-Alkylation of Secondary Amines with Alkenes". Synthesis. 1980 (4): 305–306. doi:10.1055/s-1980-29002. ISSN 0039-7881.
- Herzon, Seth B.; Hartwig, John F. (2007-05-01). "Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines". Journal of the American Chemical Society. 129 (21): 6690–6691. doi:10.1021/ja0718366. ISSN 0002-7863. PMC 2590937. PMID 17474747.
- Eisenberger, Patrick; Ayinla, Rashidat O.; Lauzon, Jean Michel P.; Schafer, Laurel L. (2009-10-19). "Tantalum–Amidate Complexes for the Hydroaminoalkylation of Secondary Amines: Enhanced Substrate Scope and Enantioselective Chiral Amine Synthesis". Angewandte Chemie International Edition. 48 (44): 8361–8365. doi:10.1002/anie.200903656. ISSN 1521-3773. PMID 19787670.
- Dörfler, Jaika; Doye, Sven (2014-05-01). "A Commercially Available Tantalum Catalyst for the Highly Regioselective Intermolecular Hydroaminoalkylation of Styrenes". European Journal of Organic Chemistry. 2014 (13): 2790–2797. doi:10.1002/ejoc.201400082. ISSN 1099-0690.
- Payne, Philippa R.; Garcia, Pierre; Eisenberger, Patrick; Yim, Jacky C.-H.; Schafer, Laurel L. (2013-05-03). "Tantalum Catalyzed Hydroaminoalkylation for the Synthesis of α- and β-Substituted N-Heterocycles". Organic Letters. 15 (9): 2182–2185. doi:10.1021/ol400729v. ISSN 1523-7060. PMID 23600625.
- Shibata, Ikuya; Kano, Takeyoshi; Kanazawa, Nobuaki; Fukuoka, Shoji; Baba, Akio (2002-04-15). "Generation of Organotantalum Reagents and Conjugate Addition to Enones". Angewandte Chemie. 114 (8): 1447–1450. doi:10.1002/1521-3773(20020415)41:8<1389::AID-ANIE1389>3.0.CO;2-D. ISSN 1521-3757. PMID 19750774.
- Yamamoto, Yoshinori; Asao, Naoki (1993-09-01). "Selective reactions using allylic metals". Chemical Reviews. 93 (6): 2207–2293. doi:10.1021/cr00022a010. ISSN 0009-2665.