O-Xylene (data page)
This page provides supplementary chemical data on o-Xylene.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as MSDS Search Engine, and follow its directions. MSDS is available from MATHESON TRI-GAS, INC. in the SDSdata.org database.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction,[1] nD | 1.5058 at 20°C |
| Abbe number | ? |
| Dielectric constant,[2] εr | 2.568 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[3] | 30.10 dyn/cm at 20°C |
| Viscosity[4] | 1.1049 mPa·s at 0°C 0.8102 mPa·s at 20°C 0.6270 mPa·s at 40°C 0.4168 mPa·s at 80°C 0.2626 mPa·s at 140°C |
| Solubility[5] | 0.171 g/L at 25°C 0.21 g/L at 45°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 247.8 K (−25.3 °C), ? Pa |
| Critical point | 631 K (358 °C), 3700 kPa |
| Std enthalpy change of fusion, ΔfusH |
13.6 kJ/mol |
| Std entropy change of fusion, ΔfusS |
54.87 J/(mol·K) at −25.3 |
| Std enthalpy change of vaporization, ΔvapH |
36.24 kJ/mol at 144.5°C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−24.4 kJ/mol |
| Standard molar entropy, S |
247 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−4552 kJ/mol |
| Heat capacity, cp | 187.0 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
19.0 kJ/mol |
| Standard molar entropy, S |
353.6 J/(mol K) |
| Heat capacity, cp | 132.5 J/(mol K) at 25°C |
| van der Waals' constants[6] | a = 3038 L2 kPa/mol2 b = 0.1755 liter per mole |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | −3.8 | 32.1 | 59.5 | 81.3 | 121.7 | 144.4 | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
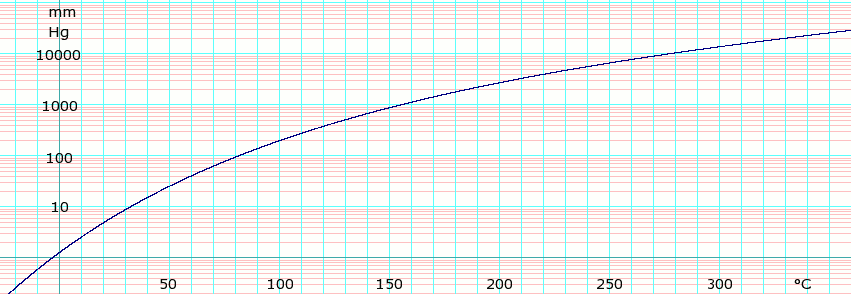
Distillation data
See also:
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
gollark: What, length terminated strings or yclib?
gollark: One of these years I should probably work out how to apply SIMD to it, for performance.
gollark: I was lazy, so it reads (can read) slightly outside of the LTS buffer.
gollark: I think `lts_length` is technically capable of causing segfaults in some situations too.
gollark: ```c#define REP(x) x x x x#define T1 "a"#define T2 REP(T1)#define T3 REP(T2)#define T4 REP(T3)#define T5 REP(T4)#define T6 REP(T5)#define T7 REP(T6)```This was part of my failed plan to implement hyperoperations in the preprocessor.
References
- Merck Index of Chemicals and Drugs, 9th ed. monograph 9743
- CRC Handbook of Chemistry and Physics, 44th ed. pp 2611–2620
- CRC Handbook of Chemistry and Physics, 44th ed. pp 2244–2248
- Lange's Handbook of Chemistry, 10th ed. pp 1669–1674
- CRC Handbook of Chemistry and Physics, 85th ed. p8-111
- Lange's Handbook of Chemistry, 10th ed, pp 1522–1524
- "Pure Component Properties" (Queriable Database). Chemical Engineering Research Information Center. Retrieved 27 May 2007.
- "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 27 May 2007.
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.