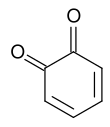
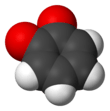
1,2-Benzoquinone
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivatives which are known.[2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexa-3,5-diene-1,2-dione[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.243.463 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H4O2 | |||
| Molar mass | 108.096 g·mol−1 | ||
| Density | 1.424 g/cm3 | ||
| Boiling point | 213.3 °C (415.9 °F; 486.4 K) | ||
| Hazards | |||
| Flash point | 76.4 °C (169.5 °F; 349.5 K) | ||
| Related compounds | |||
Related compounds |
1,4-benzoquinone quinone | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure
The molecule has C2v symmetry. X-ray crystallography shows that the double bonds are localized, with alternatingly long and short C-C distances within the ring. The C=O distances of 1.21 Å are characteristic of ketones.[3]
Preparation and occurrence
1,2-Benzoquinone is produced on oxidation of catechol exposed to air in aqueous solution[4] or by ortho oxidation of a phenol.[4]
It is a precursor to melanin.[5]
A strain of the bacterium Pseudomonas mendocina metabolises benzoic acid, yielding 1,2-benzoquinone via catechol.[6]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 728. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Liao, Chun-Chen; Peddinti, Rama Krishna (2002). "Maskedo-Benzoquinones in Organic Synthesis". Accounts of Chemical Research. 35 (10): 856–866. doi:10.1021/ar000194n. PMID 12379138.
- MacDonald, Alistair L.; Trotter, James (1973). "Crystal and molecular structure of o-benzoquinone". Journal of the Chemical Society, Perkin Transactions 2 (4): 476. doi:10.1039/P29730000476.
- Magdziak, D.; Rodriguez, A. A.; Van De Water, R. W.; Pettus, T. R. R. (2002). "Regioselective oxidation of phenols to o-quinones with o-iodoxybenzoic acid (IBX)". Org. Lett. 4 (2): 285–288. doi:10.1021/ol017068j. PMC 1557836. PMID 11796071.
- Enzymatic Browning
- Chanda Parulekar and Suneela Mavinkurve (2006). "Formation of ortho-benzoquinone from sodium benzoate by Pseudomonas mendocina P2d" (PDF). Indian Journal of Experimental Biology. 44: 157–162. PMID 16480184.