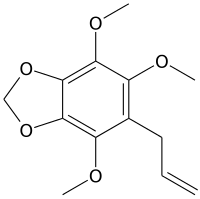
Nothoapiole
Nothoapiole is a phenylpropene, a natural organic compound present in the essential oil of Perilla frutescens from Jeju Island in Korea[1] and the major component of the essential oil obtained from the roots of Pleurospermum angelicoides Benth.[2] It is also found in the essential oil of black caraway (Carum bulbocastanum) fruits[3] and Carum nigrum.[4]
 | |
| Names | |
|---|---|
| IUPAC name
4,5,7-Trimethoxy-6-(prop-2-en-1-yl)-1,3-benzodioxole | |
| Other names
2,3,6-Trimethoxy,4,5-methylenedioxy-allylbenzene; 1,3-Benzodioxole, 4,5,7-trimethoxy-6-(2-propen-1-yl)- | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H16O5 | |
| Molar mass | 252.266 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This highly oxygenated phenylpropanoid, previously reported in a few Carum species, is structurally and biogenetically related to myristicin, apiole and dillapiole.[5][6]
See also
References
- Ito, Michiho; Toyoda, Mariko; Yuba, Akiko; Honda, Gisho (1999). "Genetic Analysis of Nothoapiol Formation in Perilla frutescens". Biological & Pharmaceutical Bulletin. 22 (6): 598–601. doi:10.1248/bpb.22.598. PMID 10408233.
- http://www.acgpubs.org/RNP/2015/Volume9/Issue%201/67-RNP-EO_1202-015.pdf
- Journal of the Science of Food and Agriculture Volume 90, Issue 3, pages 385–390, February 2010
- Singh, Gurdip; Marimuthu, Palanisamy; De Heluani, Carola S.; Catalan, Cesar A. N. (2006). "Antioxidant and Biocidal Activities of Carum nigrum(Seed) Essential Oil, Oleoresin, and Their Selected Components†". Journal of Agricultural and Food Chemistry. 54 (1): 174–81. doi:10.1021/jf0518610. PMID 16390196.
- H. Laouer; E.K. Meriem; S. Prado; N. Baldovini (2009). "An antibacterial and antifungal phenylpropanoid from Carum montanum (Coss. et Dur.) Benth. et Hook". Phytother. Res. 23 (12): 1726–1730. doi:10.1002/ptr.2820. PMID 19370550.
- M. Ito; M. Toyoda; A. Yuba; G. Honda (1999). "Genetic analysis of nothoapiole formation in Perilla frutescens". Biol. Pharm. Bull. 22 (6): 598–601. doi:10.1248/bpb.22.598. PMID 10408233.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.