Aminoxyl group
Aminoxyl radicals are chemical species containing the R2N–O• functional group. They are also known as nitroxyl radicals and nitroxides, however IUPAC discourages the use of these terms, as they erroneously suggest the presence of a nitro group.[1] They are radicals and are structurally related to hydroxylamines and N-oxoammonium salts, with which they can interconvert via a series of redox steps.
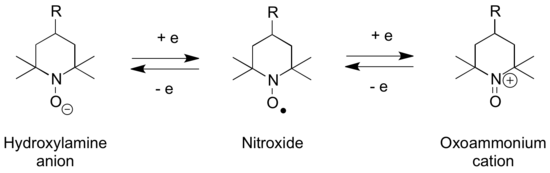
Sterically hindered aminoxyls such TEMPO and TEMPOL and are persistent (stable) radicals and find use in a range of oxoammonium-catalyzed oxidations. They are also present as transitory species various polymer stabilizers such as hindered amine light stabilizers and some p-phenylenediamine based antiozonants.[2] Nitroxide-mediated radical polymerization uses aminoxyl radicals to mediate the reaction and TEMPO-type compounds are also used as polymerization inhibitors. Various other reagents, such as N-Hydroxyphthalimide can also be converted into aminoxyl radicals as part of their chemistry.
See also
- Nitrone - structurally related, an N-oxide of an imine
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aminoxyl radicals". doi:10.1351/goldbook.A00285
- Cataldo, Franco (January 2018). "Early stages of p-phenylenediamine antiozonants reaction with ozone: Radical cation and nitroxyl radical formation". Polymer Degradation and Stability. 147: 132–141. doi:10.1016/j.polymdegradstab.2017.11.020.