Methanotroph
Methanotrophs (sometimes called methanophiles) are prokaryotes that metabolize methane as their source of carbon and energy. They can be either bacteria or archaea and can grow aerobically or anaerobically, and require single-carbon compounds to survive.
General
Methanotrophs are especially common in or near environments where methane is produced, although some methanotrophs can oxidize atmospheric methane. Their habitats include wetlands, soils, marshes, rice paddies, landfills, aquatic systems (lakes, oceans, streams) and more. They are of special interest to researchers studying global warming, as they play a significant role in the global methane budget, by reducing the amount of methane emitted to the atmosphere.[1][2]
Methanotrophy is a special case of methylotrophy, using single-carbon compounds that are more reduced than carbon dioxide. Some methylotrophs, however, can also make use of multi-carbon compounds which differentiates them from methanotrophs that are usually fastidious methane and methanol oxidizers. The only facultative methanotrophs isolated to date are members of the genus Methylocella and Methylocystis.
In functional terms, methanotrophs are referred to as methane-oxidizing bacteria, however, methane-oxidizing bacteria encompass other organisms that are not regarded as sole methanotrophs. For this reason methane-oxidizing bacteria have been separated into four subgroups: two methane-assimilating bacteria (MAB) groups, the methanotrophs, and two autotrophic ammonia-oxidizing bacteria (AAOB).[2]
Methanotroph classification
Methantrophs can be either bacteria or archaea. Which methanotroph species is present, is mainly determined by the availability of electron acceptors. Many types of methane oxidizing bacteria (MOB) are known. Differences in the method of formaldehyde fixation and membrane structure divide these bacterial methanotrophs into several groups. These include the Methylococcaceae and Methylocystaceae. Although both are included among the Proteobacteria, they are members of different subclasses. Other methanotroph species are found in the Verrucomicrobiae. Among the methanotrophic archaea, several subgroups are determined.
Aerobic methanotrophs
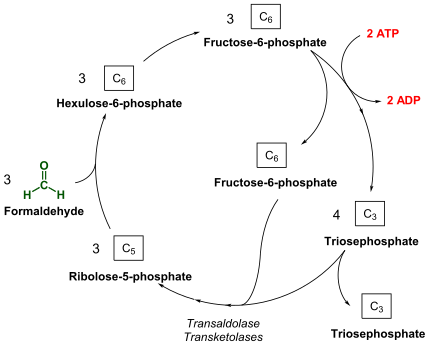
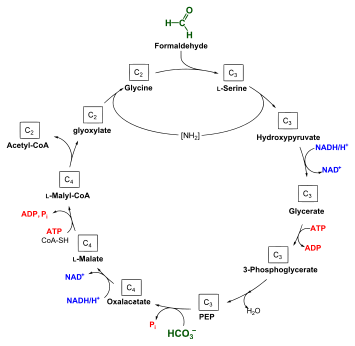
Under aerobic conditions, methanotrophs combine oxygen and methane to form formaldehyde, which is then incorporated into organic compounds via the serine pathway or the ribulose monophosphate (RuMP) pathway, and [Carbon dioxide], which is released. Type I and type X methanotrophs are part of the Gammaproteobacteria and they use the RuMP pathway to assimilate carbon. Type II methanotrophs are part of the Alphaproteobacteria and utilize the serine pathway of carbon assimilation. They also characteristically have a system of internal membranes within which methane oxidation occurs. No methanotrophic archaea are known that can use oxygen.
Anaerobic methanotrophs
Under anoxic conditions, methanotrophs use different electron acceptors for methane oxidation. This can happen in anoxic habitats such as marine or lake sediments, oxygen minimum zones, anoxic water columns, rice paddies and soils. Some specific methanotrophs can reduce nitrate or nitrite, and couple that to methane oxidation. Investigations in marine environments revealed that methane can be oxidized anaerobically by consortia of methane oxidizing archaea and sulfate-reducing bacteria. This type of anaerobic oxidation of methane (AOM) mainly occurs in anoxic marine sediments. The exact mechanism behind this is still a topic of debate but the most widely accepted theory is that the archaea use the reversed methanogenesis pathway to produce carbon dioxide and another, unknown substance. This unknown intermediate is then used by the sulfate-reducing bacteria to gain energy from the reduction of sulfate to hydrogen sulfide. The anaerobic methanotrophs are not related to the known aerobic methanotrophs; the closest cultured relative to the anaerobic methanotrophs are the methanogens in the order Methanosarcinales.[3]. Metal-oxides, such as manganese and iron, can also be used as terminal electron acceptors by ANME. For this, no consortium is needed. ANME shuttle electrons directly to the abiotic particles, which get reduced chemically [4].
In some cases, aerobic methane oxidation can take place in anoxic (no oxygen) environments. Candidatus Methylomirabilis oxyfera belongs to the phylum NC10 bacteria, and can catalyze nitrite reduction through an "intra-aerobic" pathway, in which internally produced oxygen is used to oxidise methane. In clear water lakes, methanotrophs can live in the anoxic water column, but receive oxygen from photosynthetic organisms, that they then directly consume to oxidise methane aerobically [5].
Special methanotroph species
Methylococcus capsulatus is utilised to produce animal feed from natural gas.[6]
Recently, a new bacterium Candidatus Methylomirabilis oxyfera was identified that can couple the anaerobic oxidation of methane to nitrite reduction without the need for a syntrophic partner.[7] Based on the studies of Ettwig et al.,[7] it is believed that M. oxyfera oxidizes methane anaerobically by utilizing the oxygen produced internally from the dismutation of nitric oxide into nitrogen and oxygen gas.
Methanotroph Taxonomy
Many methanotrophic cultures have been isolated and formally characterized over the past 4 decades, starting with the classical study of Whittenbury (Whittenbury et al., 1970). Currently,18 genera of cultivated aerobic methanotrophic Gammaproteobacteria and 5 genera of Alphaproteobacteria are known, represented by approx. 60 different species. [8]
Methane Oxidation by Methanotrophs
Methanotrophs oxidize methane by first initiating reduction of an oxygen atom to H2O2 and transformation of methane to CH3OH using methane monooxygenases (MMOs).[9] Furthermore, two types of MMO have been isolated from methanotrophs: soluble methane monooxygenase (sMMO) and particulate methane monooxygenase (pMMO).
Cells containing pMMO have demonstrated higher growth capabilities and higher affinity for methane than sMMO containing cells.[9] It is suspected that copper ions may play a key role in both pMMO regulation and the enzyme catalysis, thus limiting pMMO cells to more copper-rich environments than sMMO producing cells.[10]
References
- Oremland, R. S.; Culbertson, C. W. (1992). "Importance of methane-oxidizing bacteria in the methane budget as revealed by the use of a specific inhibitor". Nature. 356 (6368): 421–423. Bibcode:1992Natur.356..421O. doi:10.1038/356421a0.
- Holmes, AJ; Roslev, P; McDonald, IR; Iversen, N; Henriksen, K; Murrell, JC (1999). "Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake". Applied and Environmental Microbiology. 65 (8): 3312–8. doi:10.1128/AEM.65.8.3312-3318.1999. PMC 91497. PMID 10427012.
- Boetius, Antje; Ravenschlag, Katrin; Schubert, Carsten J.; Rickert, Dirk; Widdel, Friedrich; Gieseke, Armin; Amann, Rudolf; Jørgensen, Bo Barker; Witte, Ursula; Pfannkuche, Olaf (2000). "A marine microbial consortium apparently mediating anaerobic oxidation of methane". Nature. 407 (6804): 623–626. doi:10.1038/35036572. PMID 11034209 – via Research Gate.
- Scheller, Silvan; Yu, Hang; Chadwick, Grayson L.; McGlynn, Shawn E.; Orphan, Victoria J. (2016). "Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction". Science. 351 (6274): 703–707. doi:10.1126/science.aad7154. PMID 26912857.
- Milucka, Jana; Kirf, Mathias; Lu, Lu; Krupke, Andreas; Lam, Phyllis; Littmann, Sten; Kuypers, Marcel M.M.; Schubert, Carsten J. (Sep 2015). "Methane oxidation coupled to oxygenic photosynthesis in anoxic waters". ISME Journal. 9 (9): 1991–2002. doi:10.1038/ismej.2015.12. PMC 4542029. PMID 25679533.
- Le Page, Michael (2016-11-19). "Food made from natural gas will soon feed farm animals – and us". New Scientist. Retrieved 2016-12-11.
- Ettwig, K. F.; Butler, M. K.; Le Paslier, D.; Pelletier, E.; Mangenot, S.; Kuypers, M. M. M.; Schreiber, F.; Dutilh, B. E.; Zedelius, J.; De Beer, D.; Gloerich, J.; Wessels, H. J. C. T.; Van Alen, T.; Luesken, F.; Wu, M. L.; Van De Pas-Schoonen, K. T.; Op Den Camp, H. J. M.; Janssen-Megens, E. M.; Francoijs, K. J.; Stunnenberg, H.; Weissenbach, J.; Jetten, M. S. M.; Strous, M. (2010). "Nitrite-driven anaerobic methane oxidation by oxygenic bacteria" (PDF). Nature. 464 (7288): 543–548. Bibcode:2010Natur.464..543E. doi:10.1038/nature08883. PMID 20336137.
- Stein, Lisa Y.; Sauvageau, Dominic; Meier-Kolthoff, Jan P.; Orata, Fabini D. (2018). "Phylogenomic Analysis of the Gammaproteobacterial Methanotrophs (Order Methylococcales) Calls for the Reclassification of Members at the Genus and Species Levels". Frontiers in Microbiology. 9: 3162. doi:10.3389/fmicb.2018.03162. ISSN 1664-302X. PMC 6315193. PMID 30631317.
- Hanson, R. S.; Hanson, T. E. (1996). "Methanotrophic bacteria". Microbiological Reviews. 60 (2): 439–471. doi:10.1128/MMBR.60.2.439-471.1996. PMC 239451. PMID 8801441.
- Lieberman, R. L.; Rosenzweig, A. C. (2004). "Biological Methane Oxidation: Regulation, Biochemistry, and Active Site Structure of Particulate Methane Monooxygenase". Critical Reviews in Biochemistry and Molecular Biology. 39 (3): 147–164. doi:10.1080/10409230490475507. PMID 15596549.
External links
- Anaerobic oxidation of methane
- Methane-Eating Bug Holds Promise For Cutting Greenhouse Gas. Media Release, GNS Science, New Zealand