Methanol (data page)
This page provides supplementary chemical data on methanol.
Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Safety Datasheet (SDS) for this chemical from a reliable source such as SIRI, and follow its directions. SDS is available at MSDS, J.T. Baker and Loba Chemie
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.328 at 20 °C |
| Dielectric constant, εr | 32.66 at 20 °C |
| Magnetic susceptibility[1] | 5.3×10−7 cm3·g−1 |
| Surface tension | 22.5 dyn/cm at 20 °C |
| Viscosity[2] | 0.808 mPa·s at 0 °C 0.690 mPa·s at 10 °C 0.593 mPa·s at 20 °C 0.449 mPa·s at 40 °C 0.349 mPa·s at 60 °C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 175.5 K (−97.7 °C), 1.86·10−1 Pa[3] |
| Critical point | 513 K (240 °C), 78.5 atm |
| Std enthalpy change of fusion, ΔfusH |
3.1773 kJ/mol |
| Std entropy change of fusion, ΔfusS |
18.1 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
37.6 ± 0.5 kJ/mol[4] |
| Std entropy change of vaporization, ΔvapS |
113 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−238.4 kJ/mol |
| Standard molar entropy, S |
127.2 J/(mol K) |
| Enthalpy of combustion ΔcH |
−715.0 kJ/mol |
| Heat capacity, cp | 70.8–90.5 J/(mol K) (at −97.6 to 64.7 °C)[5]
79.9 J/(mol K) at 20 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−201.3 kJ/mol |
| Standard molar entropy, S |
239.9 J/(mol K) |
| Heat capacity,[6][7] cp | 52.29 J/(mol K) at 77 °C 61.43 J/(mol K) at 100–223 °C |
| Heat capacity ratio,[6][7] γ = cp/cv |
1.203 at 77 °C |
| van der Waals' constants[8] | a = 964.9 L2 kPa/mol2 b = 0.06702 liter per mole |
Spectral data
| UV-Vis | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||
| Major absorption bands[9] |
| ||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||
| Proton NMR | 3.97 ppm, 3.417 ppm[9] | ||||||||||||||||||||||||
| Carbon-13 NMR | 50.05 ppm[9] | ||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||
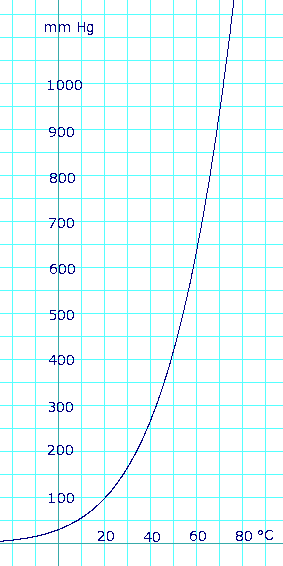
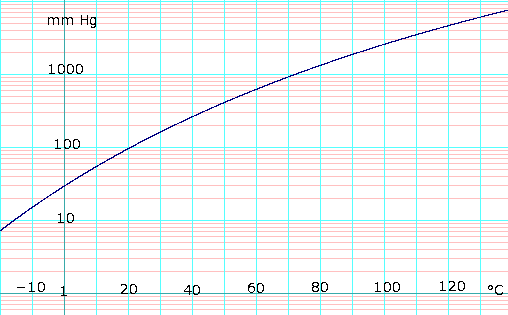
Vapor pressure of liquid
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
Here is a similar formula from the 67th edition of the CRC handbook. Note that the form of this formula as given is a fit to the Clausius–Clapeyron equation, which is a good theoretical starting point for calculating saturation vapor pressures:
- log10(P) = −(0.05223)a/T + b, where P is in mmHg, T is in kelvins, a = 38324, and b = 8.8017.
Properties of aqueous methanol solutions
Data obtained from Lange's Handbook of Chemistry, 10th ed. and CRC Handbook of Chemistry and Physics 44th ed. The annotation, d a°C/b°C, indicates density of solution at temperature a divided by density of pure water at temperature b known as specific gravity. When temperature b is 4 °C, density of water is 0.999972 g/mL.
| % wt methanol | % vol methanol | d 15.6 °C/4 °C | d 0 °C/4 °C | d 10 °C/4 °C | d 20 °C/4 °C | freezing temp °C |
|---|---|---|---|---|---|---|
| 0 | 0 | 0.99908 | 0.99984 | 0.99970 | 0.99820 | 0.0 |
| 1 | 1.25 | 0.99728 | 0.9981 | 0.9980 | 0.9965 | |
| 2 | 2.50 | 0.99543 | 0.9963 | 0.9962 | 0.9943 | |
| 3 | 3.75 | 0.99370 | 0.9946 | 0.9945 | 0.9931 | |
| 3.9 | 5 | 0.9938 | −2.2 | |||
| 4 | 4.99 | 0.99198 | 0.9930 | 0.9929 | 0.9914 | |
| 5 | 6.22 | 0.99029 | 0.9914 | 0.9912 | 0.9896 | |
| 6 | 7.45 | 0.98864 | 0.9899 | 0.9896 | 0.9880 | |
| 7 | 8.68 | 0.98701 | 0.9884 | 0.9881 | 0.9863 | |
| 8 | 9.91 | 0.98547 | 0.9870 | 0.9865 | 0.9847 | |
| 8.1 | 10 | 0.9872 | −5.0 | |||
| 9 | 11.13 | 0.98547 | 0.9856 | 0.9849 | 0.9831 | |
| 10 | 12.35 | 0.98241 | 0.9842 | 0.9834 | 0.9815 | |
| 11 | 13.56 | 0.98093 | 0.9829 | 0.9820 | 0.9799 | |
| 12 | 14.77 | 0.97945 | 0.9816 | 0.9805 | 0.9784 | |
| 12.2 | 15 | 0.9810 | −8.3 | |||
| 13 | 15.98 | 0.97802 | 0.9804 | 0.9791 | 0.9768 | |
| 14 | 17.18 | 0.97660 | 0.9792 | 0.9778 | 0.9754 | |
| 15 | 18.38 | 0.97518 | 0.9780 | 0.9764 | 0.9740 | |
| 16 | 19.58 | 0.97377 | 0.9769 | 0.9751 | 0.9725 | |
| 16.4 | 20 | 0.975 | −11.7 | |||
| 17 | 20.77 | 0.97237 | 0.9758 | 0.9739 | 0.9710 | |
| 18 | 21.96 | 0.97096 | 0.9747 | 0.9726 | 0.9696 | |
| 19 | 23.15 | 0.96955 | 0.9736 | 0.9713 | 0.9681 | |
| 20 | 24.33 | 0.96814 | 0.9725 | 0.9700 | 0.9666 | |
| 20.6 | 25 | 0.968 | −15.6 | |||
| 21 | 25.51 | 0.96673 | 0.9714 | 0.9687 | 0.9651 | |
| 22 | 26.69 | 0.96533 | 0.9702 | 0.9673 | 0.9636 | |
| 23 | 27.86 | 0.96392 | 0.9690 | 0.9660 | 0.9622 | |
| 24 | 29.03 | 0.96251 | 0.9678 | 0.9646 | 0.9607 | |
| 24.9 | 30 | 0.964 | −20.0 | |||
| 25 | 30.19 | 0.96108 | 0.9666 | 0.9632 | 0.9592 | |
| 26 | 31.35 | 0.95963 | 0.9654 | 0.9628 | 0.9576 | |
| 27 | 32.51 | 0.95817 | 0.9642 | 0.9604 | 0.9562 | |
| 28 | 33.66 | 0.95668 | 0.9629 | 0.9590 | 0.9546 | |
| 29 | 34.81 | 0.95518 | 0.9616 | 0.9575 | 0.9531 | |
| 29.2 | 35 | 0.957 | −25.0 | |||
| 30 | 35.95 | 0.95366 | 0.9604 | 0.9560 | 0.9515 | |
| 31 | 37.09 | 0.95213 | 0.9590 | 0.9546 | 0.9499 | |
| 32 | 38.22 | 0.95056 | 0.9576 | 0.9531 | 0.9483 | |
| 33 | 39.35 | 0.94896 | 0.9563 | 0.9516 | 0.9466 | |
| 33.6 | 40 | 0.950 | −30.0 | |||
| 34 | 40.48 | 0.94734 | 0.9549 | 0.9500 | 0.9450 | |
| 35 | 41.59 | 0.94570 | 0.9534 | 0.9484 | 0.9433 | |
| 36 | 42.71 | 0.94404 | 0.9520 | 0.9469 | 0.9416 | |
| 37 | 43.82 | 0.94237 | 0.9505 | 0.9453 | 0.9398 | |
| 38 | 44.92 | 0.94067 | 0.9490 | 0.9437 | 0.9381 | −35.6 |
| 39 | 46.02 | 0.93894 | 0.9475 | 0.9420 | 0.9363 | |
| 40 | 47.11 | 0.93720 | 0.9459 | 0.9403 | 0.9345 | |
| 41 | 48.20 | 0.93543 | 0.9443 | 0.9387 | 0.9327 | |
| 42 | 49.28 | 0.93365 | 0.9427 | 0.9370 | 0.9309 | |
| 43 | 50.35 | 0.93185 | 0.9411 | 0.9352 | 0.9290 | |
| 44 | 51.42 | 0.93001 | 0.9395 | 0.9334 | 0.9272 | |
| 45 | 52.49 | 0.92815 | 0.9377 | 0.9316 | 0.9252 | |
| 46 | 53.54 | 0.92627 | 0.9360 | 0.9298 | 0.9234 | |
| 47 | 54.60 | 0.92436 | 0.9342 | 0.9279 | 0.9214 | |
| 48 | 55.64 | 0.92242 | 0.9324 | 0.9260 | 0.9196 | |
| 49 | 56.68 | 0.92048 | 0.9306 | 0.9240 | 0.9176 | |
| 50 | 57.71 | 0.91852 | 0.9287 | 0.9221 | 0.9156 | |
| 51 | 58.74 | 0.91653 | 0.9269 | 0.9202 | 0.9135 | |
| 52 | 59.76 | 0.91451 | 0.9250 | 0.9182 | 0.9114 | |
| 53 | 60.77 | 0.91248 | 0.9230 | 0.9162 | 0.9094 | |
| 54 | 61.78 | 0.91044 | 0.9211 | 0.9142 | 0.9073 | |
| 55 | 62.78 | 0.90839 | 0.9191 | 0.9122 | 0.9052 | |
| 56 | 63.78 | 0.90631 | 0.9172 | 0.9101 | 0.9032 | |
| 57 | 64.77 | 0.90421 | 0.9151 | 0.9080 | 0.9010 | |
| 58 | 65.75 | 0.90210 | 0.9131 | 0.9060 | 0.8988 | |
| 59 | 66.73 | 0.89996 | 0.9111 | 0.9039 | 0.8968 | |
| 60 | 67.69 | 0.89781 | 0.9090 | 0.9018 | 0.8946 | |
| 61 | 68.65 | 0.89563 | 0.9068 | 0.8998 | 0.8924 | |
| 62 | 69.61 | 0.89341 | 0.9046 | 0.8977 | 0.8902 | |
| 63 | 70.55 | 0.89117 | 0.9024 | 0.8955 | 0.8879 | |
| 64 | 71.49 | 0.88890 | 0.9002 | 0.8933 | 0.8856 | |
| 65 | 72.42 | 0.88662 | 0.8980 | 0.8911 | 0.8834 | |
| 66 | 73.34 | 0.88433 | 0.8958 | 0.8888 | 0.8811 | |
| 67 | 74.26 | 0.88203 | 0.8935 | 0.8865 | 0.8787 | |
| 68 | 75.17 | 0.87971 | 0.8913 | 0.8842 | 0.8763 | |
| 69 | 76.08 | 0.87739 | 0.8891 | 0.8818 | 0.8738 | |
| 70 | 76.98 | 0.87507 | 0.8869 | 0.8794 | 0.8715 | |
| 71 | 77.86 | 0.87271 | 0.8847 | 0.8770 | 0.8690 | |
| 72 | 78.75 | 0.87033 | 0.8824 | 0.8747 | 0.8665 | |
| 73 | 79.62 | 0.86792 | 0.8801 | 0.8724 | 0.8641 | |
| 74 | 80.48 | 0.86546 | 0.8778 | 0.8699 | 0.8616 | |
| 75 | 81.34 | 0.86300 | 0.8754 | 0.8676 | 0.8592 | |
| 76 | 82.18 | 0.86051 | 0.8729 | 0.8651 | 0.8567 | |
| 77 | 83.02 | 0.85801 | 0.8705 | 0.8626 | 0.8542 | |
| 78 | 83.86 | 0.85551 | 0.8680 | 0.8602 | 0.8518 | |
| 79 | 84.68 | 0.85300 | 0.8657 | 0.8577 | 0.8494 | |
| 80 | 85.50 | 0.85048 | 0.8634 | 0.8551 | 0.8469 | |
| 81 | 86.31 | 0.84794 | 0.8610 | 0.8527 | 0.8446 | |
| 82 | 87.11 | 0.84536 | 0.8585 | 0.8501 | 0.8420 | |
| 83 | 87.90 | 0.84274 | 0.8560 | 0.8475 | 0.8394 | |
| 84 | 88.68 | 0.84009 | 0.8535 | 0.8449 | 0.8366 | |
| 85 | 89.45 | 0.83742 | 0.8510 | 0.8422 | 0.8340 | |
| 86 | 90.21 | 0.83475 | 0.8483 | 0.8394 | 0.8314 | |
| 87 | 90.97 | 0.83207 | 0.8456 | 0.8367 | 0.8286 | |
| 88 | 91.72 | 0.82937 | 0.8428 | 0.8340 | 0.8258 | |
| 89 | 92.46 | 0.82667 | 0.8400 | 0.8314 | 0.8230 | |
| 90 | 93.19 | 0.82396 | 0.8374 | 0.8287 | 0.8202 | |
| 91 | 93.92 | 0.82124 | 0.8347 | 0.8261 | 0.8174 | |
| 92 | 94.63 | 0.81849 | 0.8320 | 0.8234 | 0.8146 | |
| 93 | 95.33 | 0.81568 | 0.8293 | 0.8208 | 0.8118 | |
| 94 | 96.02 | 0.81285 | 0.8266 | 0.8180 | 0.8090 | |
| 95 | 96.70 | 0.80999 | 0.8240 | 0.8152 | 0.8062 | |
| 96 | 97.37 | 0.80713 | 0.8212 | 0.8124 | 0.8034 | |
| 97 | 98.04 | 0.80428 | 0.8186 | 0.8096 | 0.8005 | |
| 98 | 98.70 | 0.80143 | 0.8158 | 0.8068 | 0.7976 | |
| 99 | 99.35 | 0.79859 | 0.8130 | 0.8040 | 0.7948 | |
| 100 | 100 | 0.792 | 0.8102 | 0.8009 | 0.7917 | −97.8 |
| % wt methanol | % vol methanol | d 15.6 °C/4 °C | d 0 °C/4 °C | d 10 °C/4 °C | d 20 °C/4 °C | freezing temp °C |
Distillation data
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- NMR-002: Sample Devices and Magnetic Susceptibility.
- Lange's Handbook of Chemistry, 10th ed. pp. 1669–1674.
- http://www.ddbst.com/en/EED/PCP/VAP_C110.php
- https://webbook.nist.gov/cgi/cbook.cgi?ID=C67561&Mask=4
- GmbH, DDBST. "Molar Heat Capacity (cP) of Methanol from Dortmund Data Bank". www.ddbst.com. Retrieved 10 August 2018.
- Lange's Handbook of Chemistry 10th ed, pp. 1525–1528.
- CRC Handbook of Chemistry and Physics 44th ed. pp. 2582–2584.
- Lange's Handbook of Chemistry 10th ed, pp. 1522–1524.
- "Spectral Database for Organic Compounds". Advanced Industrial Science and Technology. Archived from the original (Queriable database) on 5 May 2006. Retrieved 9 June 2007.
- "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 7 May 2007.
- Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (sample table of physical properties)
External links
- "Properties of pure Methanol". Chemical Engineering Research Information Center. Retrieved 5 May 2007.
Except where noted otherwise, data relate to standard ambient temperature and pressure.