Melanocyte
Melanocytes are melanin-producing neural crest-derived[3] cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea),[4] the inner ear,[5] vaginal epithelium,[6] meninges,[7] bones,[8] and heart.[9] Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Functionally, melanin serves as protection against UV radiation. Melanocytes also have a role in the immune system.
| Melanocyte | |
|---|---|
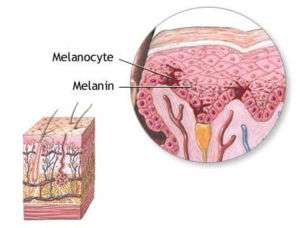 Melanocyte and melanin | |
| Details | |
| Pronunciation | /məˈlænəˌsaɪt, -noʊ-/ ( |
| Precursor | Neural crest |
| Location | Skin |
| Function | Melanin production |
| Identifiers | |
| Latin | melanocytus |
| MeSH | D008544 |
| TH | H2.00.03.0.01016 |
| FMA | 70545 |
| Anatomical terms of microanatomy | |
Function
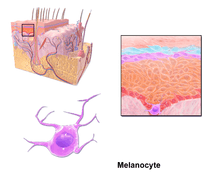
Through a process called melanogenesis, melanocytes produce melanin, which is a pigment found in the skin, eyes, hair, nasal cavity, and inner ear. This melanogenesis leads to a long-lasting pigmentation, which is in contrast to the pigmentation that originates from oxidation of already-existing melanin.
There are both basal and activated levels of melanogenesis; in general, lighter-skinned people have low basal levels of melanogenesis. Exposure to UV-B radiation causes increased melanogenesis. The purpose of melanogenesis is to protect the hypodermis, the layer under the skin, from damage by UV-B radiation. The color of the melanin is black, allowing it to absorb a majority of the UV-B light and block it from passing through the epidermis.[10]
Since the action spectrum of sunburn and melanogenesis are virtually identical, they are assumed to be induced by the same mechanism.[11] The agreement of the action spectrum with the absorption spectrum of DNA points towards the formation of cyclobutane pyrimidine dimers (CPDs) - direct DNA damage.
Typically, between 1000 and 2000 melanocytes are found per square millimeter of skin or approximately 5% to 10% of the cells in the basal layer of epidermis. Although their size can vary, melanocytes are typically 7 μm in length.
The difference in skin color between lightly and darkly pigmented individuals is due not to the number (quantity) of melanocytes in their skin, but to the melanocytes' level of activity (quantity and relative amounts of eumelanin and pheomelanin). This process is under hormonal control, including the MSH and ACTH peptides that are produced from the precursor proopiomelanocortin.
People with oculocutaneous albinism typically have a very low level of melanin production. Albinism is often but not always related to the TYR gene coding the tyrosinase enzyme. Tyrosinase is required for melanocytes to produce melanin from the amino acid tyrosine.[12] Albinism may be caused by a number of other genes as well, like OCA2,[13] SLC45A2,[14] TYRP1,[15] and HPS1[16] to name some. In all, already 17 types of oculocutaneous albinism have been recognized.[17] Each gene is related to different protein having a role in pigment production.
People with Chédiak–Higashi syndrome have a buildup of melanin granules due to abnormal function of microtubules.
Role in the immune system
In addition to their role as UV radical scavengers, melanocytes are also part of the immune system, and are considered to be immune cells.[18] Although the full role of melanocytes in immune response is not fully understood, melanocytes share many characteristics with dendritic cells: branched morphology; phagocytic capabilities; presentation of antigens to T-cells; and production and release of cytokines.[18][19][20] Although melanocytes are dendritic in form and share many characteristics with dendritic cells, they are derived from two different cell lineages. Dendritic cells, such as Langerhans cells, are derived from hematopoietic stem cells in the bone marrow. Melanocytes on the other hand originate from neural crest cells. As such, although morphologically and functionally similar, melanocytes and dendritic cells are not the same.
Melanocytes are capable of expressing MHC Class II,[19] a type of MHC expressed only by certain antigen presenting cells of the immune system, when stimulated by interactions with antigen or cytokines. All cells in any given vertebrate express MHC, but most cells only express MHC class I. The other class of MHC, Class II, is found only on "professional" antigen presenting cells such as dendritic cells, macrophages, B cells, and melanocytes. Importantly, melanocytes stimulated by cytokines express surface proteins such as CD40 and ICAM1 in addition to MHC class II, allowing for co-stimulation of T cells.[18]
In addition to presenting antigen, one of the roles of melanocytes in the immune response is cytokine production.[21] Melanocytes express many proinflammatory cytokines including IL-1, IL-3, IL-6, IL-8, TNF-α, and TGF-β.[18][19] Like other immune cells, melanocytes secrete these cytokines in response to activation of Pattern Recognition Receptors (PRRs) such as Toll Like Receptor 4 (TLR4) which recognize MAMPs. MAMPs, also known as PAMPs, are microbial associated molecular patterns, small molecular elements such as proteins, carbohydrates, and lipids present on or in a given pathogen. In addition, cytokine production by melanocytes can be triggered by cytokines secreted by other nearby immune cells.[18]
Melanocytes are ideally positioned in the epidermis to be sentinels against harmful pathogens. Melanocytes reside in the stratum basale,[21] the lowest layer of the epidermis, but they use their dendrites to interact with cells in other layers,[22] and to capture pathogens that enter the epidermis.[19] Melanocytes likely work in concert with both keratinocytes and Langerhans cells,[18][19] both of which are also actively phagocytic,[21] to contribute to the immune response.
Stimulation
Numerous stimuli are able to alter melanogenesis, or the production of melanin by cultured melanocytes, although the method by which it works is not fully understood. Certain melanocortins have been shown in laboratory testing to have effect on appetite and sexual activity in mice.[23] Eicosanoids, retinoids, oestrogens, melanocyte-stimulating hormone, endothelins, psoralens, hydantoin, forskolin, cholera toxin, isobutylmethylxanthine, diacylglycerol analogues, and UV irradiation all trigger melanogenesis and, in turn, pigmentation.[24] Increased melanin production is seen in conditions where adrenocorticotropic hormone (ACTH) is elevated, such as Addison's and Cushing's disease. This is mainly a consequence of alpha-MSH being secreted along with the hormone associated with reproductive tendencies in primates. Alpha-MSH is a cleavage product of ACTH that has an equal affinity for the MC1 receptor on melanocytes as ACTH.[25]
Melanosomes are vesicles that package the chemical inside a plasma membrane. The melanosomes are organized as a cap protecting the nucleus of the keratinocyte. When ultraviolet rays penetrate the skin and damage DNA, thymidine dinucleotide (pTpT) fragments from damaged DNA will trigger melanogenesis[26] and cause the melanocyte to produce melanosomes, which are then transferred by dendrites to the top layer of keratinocytes.
Stem cells
The precursor of the melanocyte is the melanoblast. In adults, stem cells are contained in the bulge area of the outer root sheath of hair follicles. When a hair is lost and the hair follicle regenerates, the stem cells are activated. These stem cells develop into both keratinocyte precursors and melanoblasts - and these melanoblasts supply both hair and skin (moving into the basal layer of the epidermis). There is additionally evidence that melanocyte stem cells are present in cutaneous nerves, with nerve signals causing these cells to differentiate into melanocytes for the skin.[27]
Clinical significance
See also
- Chromatophore (the pigment cell type found in poikilotherm animals)
- Eye color
- Mole (skin marking)
- Nevus depigmentosus
- Tanning activator
- List of human cell types derived from the germ layers
References
- "Melanocyte". Oxford Dictionaries UK Dictionary. Oxford University Press. Retrieved 2016-01-20.
- "Melanocyte". Merriam-Webster Dictionary.
- Cramer SF (February 1991). "The origin of epidermal melanocytes. Implications for the histogenesis of nevi and melanomas". Archives of Pathology & Laboratory Medicine. 115 (2): 115–9. PMID 1992974.
- Barden H, Levine S (June 1983). "Histochemical observations on rodent brain melanin". Brain Research Bulletin. 10 (6): 847–51. doi:10.1016/0361-9230(83)90218-6. PMID 6616275.
- Markert CL, Silvers WK (May 1956). "The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse". Genetics. 41 (3): 429–50. PMC 1209793. PMID 17247639.
- Mayeaux EJ, Cox JT, et al. (American Society for Colposcopy and Cervical Pathology) (2011-12-28). Modern Colposcopy Textbook and Atlas. Lippincott Williams & Wilkins. ISBN 9781451153835.
- Mintz B (1971). "Clonal basis of mammalian differentiation". Symposia of the Society for Experimental Biology. 25: 345–70. PMID 4940552.
- Nichols SE, Reams WM (March 1960). "The occurrence and morphogenesis of melanocytes in the connective tissues of the PET/MCV mouse strain". Journal of Embryology and Experimental Morphology. 8: 24–32. PMID 14426921.
- Theriault LL, Hurley LS (October 1970). "Ultrastructure of developing melanosomes in C57 black and pallid mice". Developmental Biology. 23 (2): 261–75. doi:10.1016/0012-1606(70)90098-9. PMID 5476812.
- Agar N, Young AR (April 2005). "Melanogenesis: a photoprotective response to DNA damage?". Mutation Research. 571 (1–2): 121–32. doi:10.1016/j.mrfmmm.2004.11.016. PMID 15748643.
- Parrish JA, Jaenicke KF, Anderson RR (August 1982). "Erythema and melanogenesis action spectra of normal human skin". Photochemistry and Photobiology. 36 (2): 187–91. doi:10.1111/j.1751-1097.1982.tb04362.x. PMID 7122713.
- "TYR". National Institutes of Health. Retrieved 23 June 2013.
- "OCA2". National Institutes of Health. Retrieved 25 March 2016.
- "SLC45A2". National Institutes of Health. Retrieved 25 March 2016.
- "TYRP1". National Institutes of Health. Retrieved 25 March 2016.
- "HPS1". National Institutes of Health. Retrieved 25 March 2016.
- Montoliu L, Grønskov K, Wei AH, Martínez-García M, Fernández A, Arveiler B, Morice-Picard F, Riazuddin S, Suzuki T, Ahmed ZM, Rosenberg T, Li W (January 2014). "Increasing the complexity: new genes and new types of albinism". Pigment Cell & Melanoma Research. 27 (1): 11–8. doi:10.1111/pcmr.12167. PMID 24066960.
- Gasque P, Jaffar-Bandjee MC (October 2015). "The immunology and inflammatory responses of human melanocytes in infectious diseases". The Journal of Infection. 71 (4): 413–21. doi:10.1016/j.jinf.2015.06.006. PMID 26092350.
- Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D, Peters E, Nordlund JJ, Abdel-Malek Z, Takeda K, Paus R, Ortonne JP, Hearing VJ, Schallreuter KU (September 2009). "What are melanocytes really doing all day long...?". Experimental Dermatology. 18 (9): 799–819. doi:10.1111/j.1600-0625.2009.00912.x. PMC 2792575. PMID 19659579.
- Mackintosh JA (July 2001). "The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin". Journal of Theoretical Biology. 211 (2): 101–13. doi:10.1006/jtbi.2001.2331. PMID 11419954.
- Abdallah F, Mijouin L, Pichon C (2017). "Skin Immune Landscape: Inside and Outside the Organism". Mediators of Inflammation. 2017: 5095293. doi:10.1155/2017/5095293. PMC 5664322. PMID 29180836.
- Tapia CV, Falconer M, Tempio F, Falcón F, López M, Fuentes M, Alburquenque C, Amaro J, Bucarey SA, Di Nardo A (July 2014). "Melanocytes and melanin represent a first line of innate immunity against Candida albicans". Medical Mycology. 52 (5): 445–54. doi:10.1093/mmy/myu026. PMID 24934806.
- Thompson C, Berger A (July 2000). "Agent provocateur pursues happiness". BMJ. 321 (7252): 12. doi:10.1136/bmj.321.7252.12. PMC 1127681. PMID 10875824.
- Roméro-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, Ballotti R (November 1996). "Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes". The Journal of Biological Chemistry. 271 (45): 28052–6. doi:10.1074/jbc.271.45.28052. PMID 8910416.
- Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ (2000-01-01). "The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation". Pigment Cell Research. 13 Suppl 8: 156–62. doi:10.1034/j.1600-0749.13.s8.28.x. PMID 11041375.
- Eller MS, Maeda T, Magnoni C, Atwal D, Gilchrest BA (November 1997). "Enhancement of DNA repair in human skin cells by thymidine dinucleotides: evidence for a p53-mediated mammalian SOS response". Proceedings of the National Academy of Sciences of the United States of America. 94 (23): 12627–32. doi:10.1073/pnas.94.23.12627. PMC 25061. PMID 9356500.
- Cichorek M, Wachulska M, Stasiewicz A, Tymińska A (February 2013). "Skin melanocytes: biology and development". Postepy Dermatologii i Alergologii. 30 (1): 30–41. doi:10.5114/pdia.2013.33376. PMC 3834696. PMID 24278043.
Further reading
- Ito S (June 2003). "The IFPCS presidential lecture: a chemist's view of melanogenesis". Pigment Cell Research. 16 (3): 230–6. doi:10.1034/j.1600-0749.2003.00037.x. PMID 12753395.
- Millington GW (May 2006). "Proopiomelanocortin (POMC): the cutaneous roles of its melanocortin products and receptors". Clinical and Experimental Dermatology. 31 (3): 407–12. doi:10.1111/j.1365-2230.2006.02128.x. PMID 16681590.
External links
| Wikimedia Commons has media related to Melanocytes. |
- Histology image: 07903loa – Histology Learning System at Boston University - "Eye: fovea, RPE"
- Histology image: 08103loa – Histology Learning System at Boston University - "Integument: pigmented skin"