Mechanostat
The Mechanostat is a term describing the way in which mechanical loading influences bone structure by changing the mass (amount of bone) and architecture (its arrangement) to provide a structure that resists habitual loads with an economical amount of material. As changes in the skeleton are accomplished by the processes of formation (bone growth) and resorption (bone loss), the mechanostat models the effect of influences on the skeleton by those processes, through their effector cells, osteocytes, osteoblasts and osteoclasts. The term was invented by Harold Frost an orthopaedic surgeon and researcher and described extensively in articles referring to Frost and Webster Jee's Utah Paradigm of Skeletal Physiology[1][2][3][4][5] in the 1960s. The Mechanostat is often defined as a practical description of Wolff's law described by Julius Wolff (1836–1902), but this is not completely accurate. Wolff wrote his treatises on bone after images of bone sections were described by Culmann and von Meyer, who suggested that the arrangement of the struts (trabeculae) at the ends of the bones were aligned with the stresses experienced by the bone. It has since been established that the static methods used for those calculations of lines of stress were inappropriate for work on what were in effect curved beams, a finding described by Lance Lanyon, a leading researcher in the area as "a triumph of a good idea over mathematics". While Wolff pulled together the work of Culmann and von Meyer, it was the French scientist Roux, who first used the term "functional adaptation" to describe the way that the skeleton optimized itself for its function, even though Wolff is credited by many for that.
According to the Mechanostat, bone growth and bone loss is stimulated by the local mechanical elastic deformation of bone. The reason for the elastic deformation of bone is the peak forces caused by muscles (e.g. measurable using mechanography). The Adaptation (feed-back control loop) of bone according to the maximum forces is considered to be a lifelong process. Hence bone adapts its mechanical properties according to the needed mechanical function – bone mass, bone geometry and hence bone strength (see also Stress-strain index, SSI) is adapted according to the everyday usage / needs. "maximal force" in this context is a simplification of the real input to bone that initiates adaptive changes. While the magnitude of a force (the weight of a load for example) is an important determinant of its effect on the skeleton, it is not the only one. The rate of application of force is also critical. Slow application of force over several seconds is not experienced by bone cells as a stimulus, but they are sensitive to very rapid application of forces (such as impacts) even of lower magnitude. High frequency vibration of bone at very low magnitudes is thought to stimulate changes but the research in the area is not completely unequivocal. It is clear that bones respond better to loading/exercise with gaps between individual events so that two loads separated by 10 seconds of rest are a more potent stimulus than 10 loads within the same 10 second period.
Due to this control loop, there is a linear relationship in the healthy body between muscle cross sectional area (as a surrogate for typical maximum forces the muscle is able to produce under physiological conditions) and the bone cross sectional area (as a surrogate for bone strength).[6][7]
These relations are of immense importance especially for bone loss situations like in osteoporosis, since an adapted training utilizing the needed maximum forces on the bone can be used to stimulate bone growth and hence prevent or help to minimize bone loss. An example for such an efficient training is vibration training or whole body vibration.
Modeling and remodeling
Frost defined four regions of elastic bone deformation which result in different consequences on the control loop:
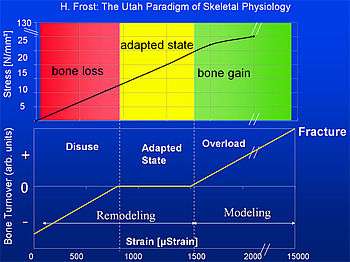
- Disuse:
Strain < circa 800μStrain: Remodeling (bone adaptation and bone repair) Bone mass and bone strength is reduced. - Adapted State:
strain between ca. 800μStrain and ca. 1500μStrain: Remodeling (bone repair) Bone mass and bone strength stays constant (homeostasis: bone resorption=bone formation) - Overload:
Strain > circa 1500μStrain: Modeling (bone growth) bone mass and bone strength is increased - Fracture:
Strain > circa 15000μStrain: maximum elastically deformation exceeded – bone fracture.
According to this a typical bone, e.g. the tibia has a security margin of about 5 to 7 between typical load (2000 to 3000 μStrain) and fracture load (about 15000μStrain).
The comments above are all one part of the way the skeleton responds to loading, because the different bones of the skeleton have a range of habituial strain environments (encompassing magnitude, rate, frequency rest periods etc) and they are not uniform. The numbers in the table are theoretical only and may reflect the response of the centre of a long bone under specific circumstances. Other parts of the same bone and other bones in the same individual experience different loading and adapt to them despite different thresholds between disuse, maintenance and adaptive formation. Furthermore bone structure is controlled by a complex series of different influences such as calcium status the effects of hormones, age, diet, sex, disease, and pharmaceuticals. A bone experiencing what would in some circumstances be seen as a stimulus to form more material could either be maintained at a constant level where circulating calcium was low, or the same loading could merely temper the amount of resorption experienced in an old person with a bone wasting disease.
Unit: Strain E
The elastic deformation of bone is measured in μStrain.[2][3] 1000μStrain = 0.1% change of length of the bone.
- Strain E at length l and change of length Δl:
It has to be considered that bone strength is highly dependent on geometry and direction of the acting forces in relation to this geometry. The fracture load for axial forces of the tibia for example is about 50 to 60 times the body weight. The fracture load for forces perpendicular to the axial direction is about 10 times lower.
Different type of bones can have different modelling and remodelling thresholds. The modeling threshold of the tibia is about 1500 μStrain (0.15% change of length), the modelling threshold for parts of the bone of the skull is quite different. Some parts of the skull such as the lower jaw (mandible) experience significant forces and strains during chewing, but the dome of the cranium for example must remain present and strong to protect the brain even if it does not experience what would be seen as stimulating strains. In one study where the strains were measured in the skull of a live human (Hillam et al J.Biomech2016) it was shown that strains in the skull never exceeded 1/10 of the peak strain in the tibia of the same individual, with similar differences in strain rates. This suggests that either bones of the skull are very sensitive to extremely low strains, or that the "genetic baseline" amount of bone in the skull in what is effectively disuse is not modified by the effects of loading. Whether the skulls of boxers are thicker than normal individuals is an intriguing question that has not been answered.
Since the physical material properties of bone (as a material) are not altered in the different bone types of the body, this difference in modelling threshold results in an increased bone mass and bone strength and hence in an increased safety factor (relation between fracture load and typical loads) for the skull compared to the tibia. A lower modeling threshold means that the same typical daily forces result in a ‘thicker’ and hence stronger bone at the skull.
Examples
Typical examples of the influence of maximum forces and the resulting elastic deformations on bone growth or bone loss are extended flights of astronauts and cosmonauts as well as patient with paraplegia due to an accident. Extended periods in free fall do not lead to loss of bone from the skull, providing support to the idea that its bone is maintained by a genetic not a mechanical influence. (Skull bone often increases in long term space flights, something thought to be related to fluid shifts within the body.) For example, a patient in a wheel chair who is using his arms but due to his paraplegia not his legs will suffer massive muscle and bone loss only in his legs due to the lack of usage of the legs. However the muscles and bones of the arms which are used every day will stay the same or might even be increased depending on the usage.[8]
The same effect can be observed for long flight Astronauts or Cosmonauts.[9] While they still use their arms in an almost normal manner due to the lack of gravity in space there are no maximum forces induced on the bones of the legs. On earth, long term players of racquet sports experience similar effects, where the dominant arm can have 30% more bone than the other due to the asymmetric applications of force.
Harold Frost applied the Mechanostat model not only to skeletal tissues but also to fibrous collagenous connective tissues, such as ligaments, tendons and fascia.[10][11] He described their adaptational responsiveness to strain in his "stretch-hypertrophy rule":
- "Intermittent stretch causes collagenous tissues to hypertrophy until the resulting increase in strength reduces elongation in tension to some minimum level".[12]
Similar to the responsiveness of bony tissues this adaptational response occurs only if the mechanical strain exceeds a certain threshold value. Harold Frost proposed that for dense collagenous connective tissues the related threshold value is around 4% strain elongation.[13]
Literature
- Frost H.M.: Defining Osteopenias and Osteoporoses: Another View (With Insights From a New Paradigm), Bone 1997, Vol. 20, No. 5, 385–391, PMID 9145234
- Frost H.M.: The Utah Paradigm of Skeletal Physiology Vol. 1, ISMNI, 1960
- Frost H.M.: The Utah Paradigm of Skeletal Physiology Vol. 2, ISMNI, 1960
- Frost H.M.: The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs, J Bone Miner Metab. 2000; 18:305–316, PMID 11052462
- Frost H.M., Schoenau E.: The muscle-bone unit in children and adolescents: an overview, 2000, J. Pediatr Endorcrinol Metab 13:571–590, PMID 10905381
- Schoenau E., NeuC.M., Beck B., Manz F., Rauch F.: Bone Mineral content per Muscle Cross-Sectional Area as an Index of the Functional Muscle-Bone Unit, J Bone Mineral Res, Vol.17, S.1095–1101, 2002, PMID 12054165
- Schießl H., Frost H.M., Jee W.S.S.: Estrogen and BoneMuscle Strength and Mass Relationships, Bone, Vol.22, S.1–6, 1998, PMID 9437507
- Eser P. et al.: Relationship between duration of paralysis and bone structure: a pQCT Study of spinal cord injured individuals, Bone, Vol.34, S.869–880, 2004, PMID 15121019
- Blottner D., Salanova M., Püttmann B., Schiffl G., Felsenberg D., Buehring B., Rittweger J.: Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest, Eur J. Appl Physiol, 2006, Vol. 97, S. 261–271, doi:10.1007/s00421-006-0160-6 PMID 16568340
- Frost, Harold "New targets for fascial, ligament and tendon research: A perspective from the Utah paradigm of skeletal physiology" J Musculoskel Neuron Interact 2003; 3(3):201–209
- Frost, Harold "The physiology of cartilagenous, fibrous, and bony tissue. C.C. Thomas, 1972
- Frost, Harold "The physiology of cartilagenous, fibrous, and bony tissue. C.C. Thomas, 1972, page 176
- Frost, Harold "Does the anterior cruciate have a modeling threshold? A case for the affirmative". J Musculoskel Neuron Interact 2001; 2(2):131–136
External links
- ISMNI – International Society of Musculoskeletal and Neuronal Interactions