MTSL
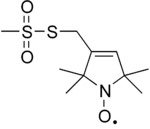
MTSL (S-(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl)methyl methanesulfonothioate) is an organosulfur compound that is used as a nitroxide spin label.[1] MTSL is bifunctional, consisting of the nitroxide and the thiosulfonate ester functional groups. The nitroxide label is sterically protected, so it is relatively unreactive.
 | |
| Names | |
|---|---|
| IUPAC name
S-(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl)methyl methanesulfonothioate | |
| Other names
MTSL | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H18NO3S2 | |
| Molar mass | 264.38 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Labeling
MTSL is attached to proteins by reaction with thiol groups. The reaction exploits standard reactivity of thiosulfate esters. Sulfinic acid (CH3SO2H) is the leaving group:
- RSO2S-nitroxide + protein-SH → protein-S-S-nitroxide + RSO2H
The heterodisulfide bond to the cysteine residue is robust, enabling site-directed spin labelling.[2][3] The MTSL moiety will add 184.3 daltons to the mass of the protein or peptide to which it is attached. The cysteine can be introduced using site-directed mutagenesis, and hence most positions in a protein can be labelled.
Spectroscopy
In Nuclear magnetic resonance the introduction of the paramagnetic group increases the relaxation rate of nearby nuclei.[1] Its presence can be detected as peak broadening and loss of intensity in peaks corresponding to nearby nuclei. Hence proximity can be inferred for all nuclei, that are affected. A major advantage of this method over traditional methods for obtaining distance restraints in protein NMR is the increased length, as paramagnetic relaxation enhancement can detect distances up to 25 Å (2.5 nm) as opposed to about 6 Å (0.6 nm) using the nuclear Overhauser effect. Spin labelling with MTSL is frequently used in investigation of residual structure in intrinsically unstructured proteins.
References
- Christian Altenbach, Kyoung-Joon Oh, René J. Trabanino, Kálmán Hideg, Wayne L. Hubbell "Estimation of Inter-Residue Distances in Spin Labeled Proteins at Physiological Temperatures: Experimental Strategies and Practical Limitations" Biochemistry, 2001, volume 40, pp 15471–15482. doi:10.1021/bi011544w
- Kenyon, G.L. and Bruice, T.W. (1977). Novel sulfhydryl reagents. Methods In Enzymology 47, 407-430. doi:10.1016/0076-6879(77)47042-3
- Berliner, L.J., Grunwald, J., Hankovszky, H.O., Hideg, K. (1982). A novel reversible thiol-specific spin label: papain active site labeling and inhibition. Analytical Biochemistry 119, 450-455. doi:10.1016/0003-2697(82)90612-1