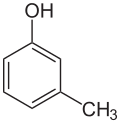
m-Cresol
meta-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and o-cresol.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylphenol | |
| Systematic IUPAC name
3-Methylbenzenol | |
| Other names
3-Cresol m-Cresol 3-Hydroxytoluene m-Cresylic acid 1-Hydroxy-3-methylbenzene | |
| Identifiers | |
3D model (JSmol) |
|
| 506719 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.253 |
| EC Number |
|
| 101411 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H8O | |
| Molar mass | 108.14 g/mol |
| Appearance | colorless liquid to yellowish liquid |
| Density | 1.034 g/cm3, liquid at 20 °C |
| Melting point | 11 °C (52 °F; 284 K) |
| Boiling point | 202.8 °C (397.0 °F; 475.9 K) |
| 2.35 g/100 ml at 20 °C 5.8 g/100 ml at 100 °C | |
| Solubility in ethanol | miscible |
| Solubility in diethyl ether | miscible |
| Vapor pressure | 0.14 mmHg (20°C)[1] |
| -72.02·10−6 cm3/mol | |
Refractive index (nD) |
1.5398 |
| Viscosity | 6.1 cP at 40 °C |
| Hazards | |
| Main hazards | May cause serious burns. Very destructive of mucous membranes. Harmful if inhaled. Toxic in contact with the skin or if swallowed. |
| Safety data sheet | External MSDS |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H227, H301, H311, H314, H318, H351, H370, H372, H373, H401 |
| P201, P202, P210, P260, P264, P270, P273, P280, P281, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P307+311, P308+313, P310, P312, P314, P321, P322, P330, P361, P363 | |
| NFPA 704 (fire diamond) | |
| Flash point | 86 °C |
| Explosive limits | 1.1%-? (149°C)[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
242 mg/kg (oral, rat, 1969) 2020 mg/kg (oral, rat, 1944) 828 mg/kg (oral, mouse)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 5 ppm (22 mg/m3) [skin][1] |
REL (Recommended) |
TWA 2.3 ppm (10 mg/m3)[1] |
IDLH (Immediate danger) |
250 ppm[1] |
| Related compounds | |
Related phenols |
o-cresol, p-cresol, phenol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Together with many other compounds, m-cresol is traditionally extracted from coal tar, the volatile materials obtained in the production of coke from (bituminous) coal. This residue contains a few percent by weight of phenol and isomeric cresols. In the cymene-cresol process, toluene is alkylated with propene to give isomers of cymene, which can be oxidatively dealkylated analogous to the cumene process. Another method, involves carbonylation of a mixture of methallyl chloride and acetylene in the presence of nickel carbonyl.[3]
Applications
m-Cresol is a precursor to numerous compounds. Important examples include:
- pesticides such as fenitrothion and fenthion
- synthetic vitamin E by methylation to give the 2,3,6-trimethylphenol[3]
- antiseptics, such as amylmetacresol
- a solvent for polymers. For example, polyaniline is cast from a solution of m-cresol to form a polyaniline film with a superior conductivity than polyaniline alone. This phenomenon is known as secondary doping.[4]
- preservatives in some insulins.
- the starting point in the total synthesis of thymol,[5] an important synthetic chemical for regions lacking natural sources of the flavor compound:[6]
- the synthesis of dicresulene and policresulen.
- the synthesis of toliprolol, tolamolol & cresatin [122-46-3]
Natural occurrences
m-Cresol is a component found in temporal glands secretions during musth in male African elephants (Loxodonta africana).[7]
m-Cresol is a constituent of tobacco smoke.[8]
See also
References
- NIOSH Pocket Guide to Chemical Hazards. "#0155". National Institute for Occupational Safety and Health (NIOSH).
- "Cresol (o, m, p isomers)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Helmut Fiege (2007). "Cresols and Xylenols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_025.
- Alan G. MacDiarmid and Arthur J. Epstein. 1995. "Secondary Doping in Polyaniline" Synthetic Metals 69 (85-92).
- Stroh, R.; Sydel, R.; Hahn, W. (1963). Foerst, Wilhelm (ed.). Newer Methods of Preparative Organic Chemistry, Volume 2 (1st ed.). New York: Academic Press. p. 344. ISBN 9780323150422.
- Asim Kumar Mukhopadhyay (2004). Industrial Chemical Cresols and Downstream Derivatives. New York: CRC Press. pp. 99–100. ISBN 9780203997413.
- Some chemical constituents of the secretion from the temporal gland of the African elephant (Loxodonta africana). Jack Adams, Alexander Garcia and Christopher S. Foote, Journal of Chemical Ecology, 1978, Volume 4, Number 1, 17-25, doi:10.1007/BF00988256
- Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
External links
- NIOSH Pocket Guide to Chemical Hazards cdc.gov
- Chemical and physical properties chemicalbook.com
