Lemieux–Johnson oxidation
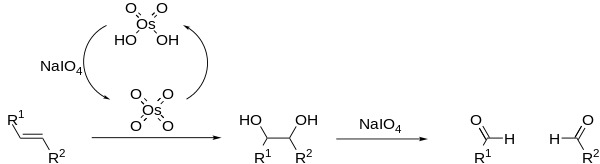
The Lemieux–Johnson or Malaprade–Lemieux–Johnson oxidation is a chemical reaction in which an olefin undergoes oxidative cleavage to form two aldehyde or ketone units. The reaction is named after its inventors, Raymond Urgel Lemieux and William Summer Johnson, who published it in 1956.[1] The reaction proceeds in a two step manner, beginning with dihydroxylation of the alkene by osmium tetroxide, followed by a Malaprade reaction to cleave the diol using periodate.[2] Excess periodate is used to regenerate the osmium tetroxide, allowing it to be used in catalytic amounts. The Lemieux–Johnson reaction ceases at the aldehyde stage of oxidation and therefore produces the same results as ozonolysis.
The classical Lemieux–Johnson oxidation often generates many side products, resulting in low reaction yields; however the addition of non-nucleophilic bases, such as 2,6-lutidine, can improve on this.[3] OsO4 may be replaced with a number of other Osmium compounds.[4][5] Periodate may also be replaced with other oxidising agents, such as oxone.[6]
History
The development of the Lemieux–Johnson oxidation was preceded by an analogous process, developed by Lemieux, which used an aqueous solution of sodium periodate with a low (catalytic) concentration of potassium permanganate.[7] This mixture became known as Lemieux reagent[8][9] and has been used to determine the position of double bonds and for preparing carbonyl compounds.[10] Unlike the Lemieux–Johnson oxidation, which normally stops at the aldehyde, this older method could continue to give a mixture of aldehydes and carboxylic acids.[1]
References
- Pappo, R.; Allen, D. S., Jr.; Lemieux, R. U.; Johnson, W. S. (1956). "Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds". J. Org. Chem. 21 (4): 478–479. doi:10.1021/jo01110a606.CS1 maint: multiple names: authors list (link)
- Hassner, Alfred; Stumer, C. (2002). "Malaprade–Lemieux–Johnson". Organic Syntheses Based on Name Reactions. Elsevier. p. 227.
- Hua, Z.; Yu, W.; Jin, Z. (2004). "An Improved Procedure for the Oxidative Cleavage of Olefins by OsO4-NaIO4". Org. Lett. 6 (19): 3217. doi:10.1021/ol0400342.
- Whitehead, Daniel C.; Travis, Benjamin R.; Borhan, Babak. "The OsO4-mediated oxidative cleavage of olefins catalyzed by alternative osmium sources". Tetrahedron Letters. 47 (22): 3797–3800. doi:10.1016/j.tetlet.2006.03.087.
- Kim, Seyoung; Chung, Jooyoung; Kim, B. Moon. "Recycling of osmium catalyst in oxidative olefin cleavage: a chemoentrapment approach". Tetrahedron Letters. 52 (12): 1363–1367. doi:10.1016/j.tetlet.2011.01.065.
- Travis, Benjamin R.; Narayan, Radha S.; Borhan, Babak. "Osmium Tetroxide-Promoted Catalytic Oxidative Cleavage of Olefins: An Organometallic Ozonolysis". Journal of the American Chemical Society. 124 (15): 3824–3825. doi:10.1021/ja017295g.
- Lemieux, R. U.; Rudloff, E. Von (November 1955). "PERIODATE–PERMANGANATE OXIDATIONS: I. OXIDATION OF OLEFINS". Canadian Journal of Chemistry. 33 (11): 1701–1709. doi:10.1139/v55-208.
- Ho, Tse-Lok; Fieser, Mary; Fieser, Louis (2006). "Sodium periodate-Potassium permanganate (Lemieux-von Rudloff reagent)". doi:10.1002/9780471264194.fos09311. Cite journal requires
|journal=(help) - Wee, A. G.; Liu, B (15 Apr 2001). "Sodium Periodate–Potassium Permanganate". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rs096.
- Lemieux, R. U.; von Rudloff, E. (1955). "Periodate-permanganate Oxidation: II. DETERMINATION OF TERMINAL METHYLENE GROUPS". Canadian Journal of Chemistry. 33 (11): 1710–1713. doi:10.1139/v55-209.