LMO2
LIM domain only 2 (rhombotin-like 1), also known as LMO2, RBTNL1, RBTN2, RHOM2, LIM Domain Only Protein 2, TTG2, and T-Cell Translocation Protein 2, is a protein which in humans is encoded by the LMO2 gene.[5]
Function
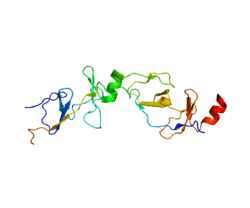
LMO2 encodes a cysteine-rich, two LIM domain protein that is required for yolk sac erythropoiesis.[6] The LMO2 protein has a central and crucial role in hematopoietic development and is highly conserved.
Clinical significance
The LMO2 transcription start site is located approximately 25 kb downstream from the 11p13 T-cell translocation cluster (11p13 ttc), where a number of T-cell acute lymphoblastic leukemia-specific translocations occur.[7]
Interactions
LMO2 has been shown to interact with:
gollark: I have enough dice.
gollark: I could make multiplayer infinitely large multidimensional tic-tac-toe.
gollark: In general.
gollark: I do not want to.
gollark: No.
References
- GRCh38: Ensembl release 89: ENSG00000135363 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000032698 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH (May 1991). "The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13". Proceedings of the National Academy of Sciences of the United States of America. 88 (10): 4367–71. doi:10.1073/pnas.88.10.4367. PMC 51660. PMID 2034676.
- Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH (Jul 1994). "The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development". Cell. 78 (1): 45–57. doi:10.1016/0092-8674(94)90571-1. PMID 8033210.
- EntrezGene 4005
- Osada H, Grutz G, Axelson H, Forster A, Rabbitts TH (Oct 1995). "Association of erythroid transcription factors: complexes involving the LIM protein RBTN2 and the zinc-finger protein GATA1". Proceedings of the National Academy of Sciences of the United States of America. 92 (21): 9585–9. doi:10.1073/pnas.92.21.9585. PMC 40846. PMID 7568177.
- Mao S, Neale GA, Goorha RM (Apr 1997). "T-cell oncogene rhombotin-2 interacts with retinoblastoma-binding protein 2". Oncogene. 14 (13): 1531–9. doi:10.1038/sj.onc.1200988. PMID 9129143.
- Bégay-Müller V, Ansieau S, Leutz A (Jun 2002). "The LIM domain protein Lmo2 binds to AF6, a translocation partner of the MLL oncogene". FEBS Letters. 521 (1–3): 36–8. doi:10.1016/s0014-5793(02)02814-4. PMID 12067721.
- Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH, Baer R (Oct 1994). "Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia". The EMBO Journal. 13 (20): 4831–9. doi:10.1002/j.1460-2075.1994.tb06809.x. PMC 395422. PMID 7957052.
- Valge-Archer VE, Osada H, Warren AJ, Forster A, Li J, Baer R, Rabbitts TH (Aug 1994). "The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells". Proceedings of the National Academy of Sciences of the United States of America. 91 (18): 8617–21. doi:10.1073/pnas.91.18.8617. PMC 44657. PMID 8078932.
- Goardon N, Lambert JA, Rodriguez P, Nissaire P, Herblot S, Thibault P, Dumenil D, Strouboulis J, Romeo PH, Hoang T (Jan 2006). "ETO2 coordinates cellular proliferation and differentiation during erythropoiesis". The EMBO Journal. 25 (2): 357–66. doi:10.1038/sj.emboj.7600934. PMC 1383517. PMID 16407974.
Further reading
- Royer-Pokora B, Loos U, Ludwig WD (Oct 1991). "TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11)". Oncogene. 6 (10): 1887–93. PMID 1923511.
- Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH (May 1991). "The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13". Proceedings of the National Academy of Sciences of the United States of America. 88 (10): 4367–71. doi:10.1073/pnas.88.10.4367. PMC 51660. PMID 2034676.
- Boehm T, Spillantini MG, Sofroniew MV, Surani MA, Rabbitts TH (May 1991). "Developmentally regulated and tissue specific expression of mRNAs encoding the two alternative forms of the LIM domain oncogene rhombotin: evidence for thymus expression". Oncogene. 6 (5): 695–703. PMID 2052354.
- Dong WF, Xu Y, Hu QL, Munroe D, Minowada J, Housman DE, Minden MD (Nov 1995). "Molecular characterization of a chromosome translocation breakpoint t(11;14)(p13;q11) from the cell line KOPT-K1". Leukemia. 9 (11): 1812–7. PMID 7475267.
- Osada H, Grutz G, Axelson H, Forster A, Rabbitts TH (Oct 1995). "Association of erythroid transcription factors: complexes involving the LIM protein RBTN2 and the zinc-finger protein GATA1". Proceedings of the National Academy of Sciences of the United States of America. 92 (21): 9585–9. doi:10.1073/pnas.92.21.9585. PMC 40846. PMID 7568177.
- Sánchez-García I, Axelson H, Rabbitts TH (Apr 1995). "Functional diversity of LIM proteins: amino-terminal activation domains in the oncogenic proteins RBTN1 and RBTN2". Oncogene. 10 (7): 1301–6. PMID 7731680.
- Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH, Baer R (Oct 1994). "Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia". The EMBO Journal. 13 (20): 4831–9. doi:10.1002/j.1460-2075.1994.tb06809.x. PMC 395422. PMID 7957052.
- Valge-Archer VE, Osada H, Warren AJ, Forster A, Li J, Baer R, Rabbitts TH (Aug 1994). "The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells". Proceedings of the National Academy of Sciences of the United States of America. 91 (18): 8617–21. doi:10.1073/pnas.91.18.8617. PMC 44657. PMID 8078932.
- Wilkinson DA, Neale GA, Mao S, Naeve CW, Goorha RM (Jan 1997). "Elf-2, a rhombotin-2 binding ets transcription factor: discovery and potential role in T cell leukemia". Leukemia. 11 (1): 86–96. doi:10.1038/sj.leu.2400516. PMID 9001422.
- Mao S, Neale GA, Goorha RM (Apr 1997). "T-cell oncogene rhombotin-2 interacts with retinoblastoma-binding protein 2". Oncogene. 14 (13): 1531–9. doi:10.1038/sj.onc.1200988. PMID 9129143.
- Osada H, Grutz GG, Axelson H, Forster A, Rabbitts TH (Apr 1997). "LIM-only protein Lmo2 forms a protein complex with erythroid transcription factor GATA-1". Leukemia. 11 Suppl 3: 307–12. PMID 9209374.
- Wadman IA, Osada H, Grütz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH (Jun 1997). "The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins". The EMBO Journal. 16 (11): 3145–57. doi:10.1093/emboj/16.11.3145. PMC 1169933. PMID 9214632.
- Visvader JE, Mao X, Fujiwara Y, Hahm K, Orkin SH (Dec 1997). "The LIM-domain binding protein Ldb1 and its partner LMO2 act as negative regulators of erythroid differentiation". Proceedings of the National Academy of Sciences of the United States of America. 94 (25): 13707–12. doi:10.1073/pnas.94.25.13707. PMC 28370. PMID 9391090.
- Jurata LW, Pfaff SL, Gill GN (Feb 1998). "The nuclear LIM domain interactor NLI mediates homo- and heterodimerization of LIM domain transcription factors". The Journal of Biological Chemistry. 273 (6): 3152–7. doi:10.1074/jbc.273.6.3152. PMID 9452425.
- Kenny DA, Jurata LW, Saga Y, Gill GN (Sep 1998). "Identification and characterization of LMO4, an LMO gene with a novel pattern of expression during embryogenesis". Proceedings of the National Academy of Sciences of the United States of America. 95 (19): 11257–62. doi:10.1073/pnas.95.19.11257. PMC 21629. PMID 9736723.
- Ono Y, Fukuhara N, Yoshie O (Dec 1998). "TAL1 and LIM-only proteins synergistically induce retinaldehyde dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia by acting as cofactors for GATA3". Molecular and Cellular Biology. 18 (12): 6939–50. doi:10.1128/MCB.18.12.6939. PMC 109277. PMID 9819382.
- Bach I, Rodriguez-Esteban C, Carrière C, Bhushan A, Krones A, Rose DW, Glass CK, Andersen B, Izpisúa Belmonte JC, Rosenfeld MG (Aug 1999). "RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex". Nature Genetics. 22 (4): 394–9. doi:10.1038/11970. PMID 10431247.
- Vitelli L, Condorelli G, Lulli V, Hoang T, Luchetti L, Croce CM, Peschle C (Jul 2000). "A pentamer transcriptional complex including tal-1 and retinoblastoma protein downmodulates c-kit expression in normal erythroblasts". Molecular and Cellular Biology. 20 (14): 5330–42. doi:10.1128/MCB.20.14.5330-5342.2000. PMC 85982. PMID 10866689.
- Davenport J, Neale GA, Goorha R (Nov 2000). "Identification of genes potentially involved in LMO2-induced leukemogenesis". Leukemia. 14 (11): 1986–96. doi:10.1038/sj.leu.2401913. PMID 11069036.
- Sum EY, Peng B, Yu X, Chen J, Byrne J, Lindeman GJ, Visvader JE (Mar 2002). "The LIM domain protein LMO4 interacts with the cofactor CtIP and the tumor suppressor BRCA1 and inhibits BRCA1 activity". The Journal of Biological Chemistry. 277 (10): 7849–56. doi:10.1074/jbc.M110603200. PMID 11751867.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.