Jennite
Jennite is a calcium silicate hydrate mineral of general chemical formula: Ca9Si6O18(OH)6·8H2O.
| Jennite | |
|---|---|
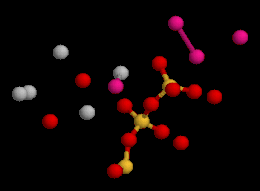 Crystal structure of jennite: elementary unit cell viewed in 3D | |
| General | |
| Category | Silicate mineral |
| Formula (repeating unit) | Ca9Si6O18(OH)6·8H2O |
| Strunz classification | 9.DG.20 |
| Crystal system | Triclinic |
| Crystal class | Pinacoidal (1) (same H-M symbol) |
| Space group | P1 |
| Unit cell | a = 10.56, b = 7.25 c = 10.81 [Å]; α = 99.7° β = 97.67°, γ = 110.07°; Z = 1 |
| Identification | |
| Formula mass | 1,063 g/mol |
| Color | White |
| Crystal habit | Blade shaped crystals, fibrous aggregates, platy - sheet forms |
| Cleavage | Distinct on [001] |
| Mohs scale hardness | 3.5 |
| Luster | Vitreous (glassy) |
| Streak | White |
| Diaphaneity | Transparent to translucent |
| Density | 2.32 – 2.33 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.548 - 1.552 nβ = 1.562 - 1.564 nγ = 1.570 - 1.571 |
| Birefringence | δ = 0.022 |
| 2V angle | Measured: 74° |
| Ultraviolet fluorescence | Weak white |
| References | [1][2][3][4] |
Jennite occurs as an alteration mineral in metamorphosed limestone and skarn.[2] It typically occurs as vein and open space fillings as a late mineral phase.[4] It also occurs in hydrated cement paste.
A first specimen of jennite found in 1966 at the Crestmore quarries (Crestmore, Riverside County, California, US) was analysed and identified as a new mineral by Carpenter in 1966 (Carpenter, 1966). They named it in honor of its discoverer: Clarence Marvin Jenni (1896–1973) director of the Geological Museum at the University of Missouri.[2]
In contrast to the first analysis made by Carpenter, jennite does not contain appreciable amount of sodium when the Crestmore specimen was reexamined (Gard, 1977).
The structure of jennite is made of three distinct modules: ribbons of edge-sharing calcium octahedra, silicate chains of wollastonite-type running along the b axis, and additional calcium octahedra on inversion centers. The hydroxyl groups are bonded to three calcium cations while no SiOH groups are observed (Bonaccorsi, 2004).
Jennite transforms to metajennite at 70–90 °C (158–194 °F) by losing four water molecules (Gard, 1977).
Cement chemistry
Jennite is often used in thermodynamical calculations to represent the pole of the less evolved calcium silicate hydrate (C-S-H). The value of its Ca/Si or CaO/SiO2 (C/S) ratio is 1.5 (9/6). Tobermorite represents the more evolved pole with a C/S ratio of 0.83 (5/6).
See also
- Other calcium silicate hydrate (C-S-H) minerals:
- Other calcium aluminium silicate hydrate, (C-A-S-H) minerals:
References
- Bibliography
- Abdul-Jaber, Q.H.; Khoury, H. (1998), "Unusual mineralisation in the Maqarin Area (North Jordan) and the occurrence of some rare minerals in the marbles and the weathered rocks", Neues Jahrb. Geol. Paläontol. Abh., 208, pp. 603–629
- Bonaccorsi, E.; Merlino, S.; Taylor, H.F.W. (2004), "The crystal structure of jennite, Ca9Si6O18(OH)6 · 8 H2O, Locality: Fuka, Japan", Cement and Concrete Research, 34 (9), pp. 1481–1488, doi:10.1016/j.cemconres.2003.12.033, retrieved 2009-02-04
- Carpenter, A.B.; Chalmers, R.A.; Gard, J.A.; Speakman, K.; Taylor, H.F.W. (1966), "Jennite, a new mineral" (PDF), American Mineralogist, 51, pp. 56–74, retrieved 2009-02-04
- Gard, J.A.; Taylor, H.F.W.; Cliff, G.; Lorimer, G.W. (1977), "A reexamination of jennite" (PDF), American Mineralogist, 62, pp. 365–368, retrieved 2009-02-04
Further reading
- Chen, Jeffrey J.; Jeffrey J. Thomas; Hal F.W. Taylor; Hamlin M. Jennings (2004), "Solubility and structure of calcium silicate hydrate", Cement and Concrete Research, 34 (9): 1499–1519, CiteSeerX 10.1.1.568.4216, doi:10.1016/j.cemconres.2004.04.034, ISSN 0008-8846
- Eakle, Arthur S. (1927), "Famous mineral localities: Crestmore, Riverside County, California", American Mineralogist, 12: 319–321, retrieved 2009-11-01
- Naomichi, Hara (2000), "Formation of jennite and tobermorite from amorphous silica", J. Soc. Inorg. Mater. Japan, 7 (285): 133–142, ISSN 1345-3769, retrieved 2009-02-04
- Merlino, S.; Bonaccorsi E.; Armbruster T. (2001), "The real structure of tobermorite 11A: normal and anomalous forms, OD character and polytypic modifications (Note: MDO2 - synchrotron radiation source. Locality: Bascenov, Urals, Russia)", European Journal of Mineralogy, 13 (3): 577–590, Bibcode:2001EJMin..13..577M, doi:10.1127/0935-1221/2001/0013-0577
| Wikimedia Commons has media related to Jennite. |