Isomaltase
Isomaltase (EC 3.2.1.10) is an enzyme that breaks the bonds linking saccharides, which cannot be broken by amylase or maltase. It digests polysaccharides at the alpha 1-6 linkages. Its substrate, alpha-limit dextrin, is a product of amylopectin digestion that retains its 1-6 linkage (its alpha 1-4 linkages having already been broken down by amylase). The product of the enzymatic digestion of alpha-limit dextrin by isomaltase is maltose.
| Oligo-1,6-glucosidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 3.2.1.10 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Isomaltase helps amylase to digest alpha-limit dextrin to produce maltose. The human sucrase-isomaltase is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase.
Nomenclature
The systematic name of sucrase-isomaltase is oligosaccharide 6-alpha-glucohydrolase. This enzyme is also known as:
- Sucrase-alpha-dextrinase
- oligo-1,6-glucosidase,
- limit dextrine,
- so maltase,
- exo-oligo-1,6-glucosidase,
- dextrin 6alpha-glucanohydrolase,
- alpha-limit dextrine,
- dextrin 6-glucanohydrolase, and
- oligosaccharide alpha-1,6-glucohydrolase.
Mechanism
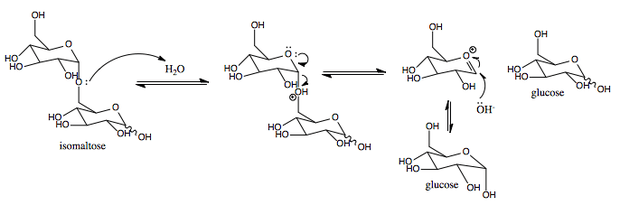
This enzyme catalyses the following chemical reaction
- Hydrolysis of (1->6)-alpha-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by enzyme EC 3.2.1.1.
Hydrolysis uses water to cleave chemical bonds. Sucrase-isomaltase’s mechanism results in a net retention of configuration at the anomeric center.[1]
External links
- Isomaltase at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- Sim L, Willemsma C, Mohan S, Naim HY, Pinto BM, Rose DR (June 2010). "Structural basis for substrate selectivity in human maltase-glucoamylase and sucrase-isomaltase N-terminal domains". The Journal of Biological Chemistry. 285 (23): 17763–70. doi:10.1074/jbc.M109.078980. PMC 2878540. PMID 20356844.