Hydrovinylation
In organic chemistry, hydrovinylation is the formal insertion of an alkene into the C-H bond of ethylene. The more general reaction, hydroalkenylation is the formal insertion of an alkene into the C-H bond of any terminal alkene. The reaction is catalyzed by metal complexes. A representative reaction is the conversion of styrene and ethylene to 3-phenybutene:[1]
- PhCH=CH2 + CH2=CH2 → CH3-CH(Ph)-CH=CH2.
Ethylene dimerization
The dimerization of ethylene gives 1-butene is another example of a hydrovinylation. In the Dimersol and Alphabutol Processes, alkenes are dimerized for the production of gasoline and for comonomers such as 1-butene. These processes operate at several refineries across the world at the scales of about 400,000 tons/year (2006 report).[2] 1-Butene is amenable to isomerization to 2-butenes, which is used in Olefin conversion technology to give propylene.
Hydroarylation
Hydroarylation is again a special case of hydrovinylation. Hydroarylation has been demonstrated for alkyne and alkene substrates. An early example was provided by the Murai reaction, which involves the insertion of alkenes into a C-H bond of acetophenone. The keto group directs the regiochemistry, stabilizing an aryl intermediate.[3]
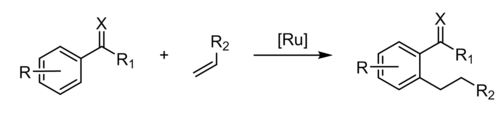
When catalyzed by palladium carboxylates, a key step is electrophilic aromatic substitution to give a Pd(II) aryl intermediate.[4]
In organic synthesis
As first reported by Alderson, Jenner, and Lindsey hydrovinylation uses rhodium- and ruthenium-based catalysts. Catalysts based on iron, cobalt, nickel, and palladium have also been demonstrated. The addition can be done highly regio- and stereoselectively, the choices of metal centers, ligands, substrates and counterions often play very important role.[5][6][7] N-heterocyclic carbene complexes of Ni allow the selective preparations of functionalized geminal olefins or 1,1-disubstituted alkenes.[8][9]
References
- T. V. RajanBabu, G. A. Cox (2014). "5.32 Hydrovinylation Reactions in Organic Synthesis". Hydrovinylation Reactions in Organic Synthesis. Comprehensive Organic Synthesis II (Second Edition). 5. pp. 1582–1620. doi:10.1016/B978-0-08-097742-3.00533-4. ISBN 9780080977430.CS1 maint: uses authors parameter (link)
- Yves Chauvin (2006). "Olefin Metathesis: The Early Days (Nobel Lecture)". Angew. Chem. Int. Ed. 45 (23): 3740–3747. doi:10.1002/anie.200601234. PMID 16724296.
- Murai, Shinji; Kakiuchi, Fumitoshi; Sekine, Shinya; Tanaka, Yasuo; Kamatani, Asayuki; Sonoda, Motohiro; Chatani, Naoto (1993-12-09). "Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins". Nature. 366 (6455): 529–531. Bibcode:1993Natur.366..529M. doi:10.1038/366529a0.
- Jia, C.; Kitamura, T.; Fujiwara, Y. (2001). "Catalytic Functionalization of Arenes and Alkanes Via C-H Bond Activation". Acc. Chem. Res. 34 (8): 633–639. doi:10.1021/ar000209h. PMID 11513570.CS1 maint: uses authors parameter (link)
- Grutters, M. M. P.; Muller, C.; Vogt, D. (2006). "Highly Selective Cobalt-Catalyzed Hydrovinylation of Styrene". J. Am. Chem. Soc. 128 (23): 7414–5. doi:10.1021/ja058095y. PMID 16756275.
- Hilt, G.; Danz, M.; Treutwein, J. (2009). "Cobalt-Catalyzed 1,4-Hydrovinylation of Styrenes and 1-Aryl-1,3-butadienes". Org. Lett. 11 (15): 3322–5. doi:10.1021/ol901064p. PMID 19583205.
- Sharma, R. K.; RajanBabu, T. V. (2010). "Asymmetric Hydrovinylation of Unactivated Linear 1,3-Dienes". J. Am. Chem. Soc. 132 (10): 3295–7. doi:10.1021/ja1004703. PMC 2836389. PMID 20163120.
- Ho, C.-Y.; He, L. (2010). "Catalytic Intermolecular Tail-to-Tail Hydroalkenylation of Styrenes with alpha-Olefins: Regioselective Migratory Insertion Controlled by a Nickel/N-Heterocyclic Carbene". Angew. Chem. Int. Ed. 49 (48): 9182–9186. doi:10.1002/anie.201001849. PMID 20853303.
- Ho, C.-Y.; He, L. (2012). "Shuffle Off the Classic Beta-Si Elimination by Ni-NHC Cooperation: Implication for C–C Forming Reactions Involving Ni-Alkyl-Beta-Silanes". Chem. Commun. 48 (10): 1481–1483. doi:10.1039/c1cc14593b. PMID 22116100.