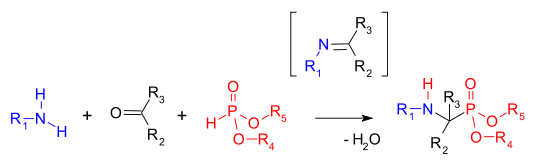
Hydrophosphonylation
In chemistry hydrophosphonylation refers to any reaction where addition across a double bond generates a phosphonate (RP(O)(OR')2) group.[1] Examples include the Kabachnik–Fields reaction, where a dialkylphosphite reacts across an imine to form an aminophosphonate. The reaction is catalyzed by bases and is subject to organocatalysis.[2][3] Important compounds generated by this reaction include the common herbicide glyphosate.
Hydrophosphonylation reactions
gollark: Right. Yes. That.
gollark: ++tel init_webhook
gollark: ++tel link apionet `#a`
gollark: Yes there is.
gollark: I manually fixed the fallback, as it was missing the guild ID.
See also
- Hydrophosphination - the addition of a phosphine derivative (PHR2) across a double bond
References
- Pudovik, A. N.; Konovalova, I. V. (1979). "Addition Reactions of Esters of Phosphorus(III) Acids with Unsaturated Systems". Synthesis. 1979 (02): 81–96. doi:10.1055/s-1979-28566.
- Pedro Merino, Eugenia Marqués‐López, Raquel P. Herrera (2008). "Catalytic Enantioselective Hydrophosphonylation of Aldehydes and Imines". Advanced Synthesis and Catalysis. 350: 1195–1208. doi:10.1002/adsc.200800131. hdl:10261/114023.CS1 maint: uses authors parameter (link)
- Shaoquan Lin, Yasunari Otsuka, Liang Yin, Naoya Kumagai, Masakatsu Shibasaki (2017). "Catalytic Enantioselective Addition of Diethyl Phosphite to N-Thiophosphinoyl Ketimines: Preparation of (R)-Diethyl (1-Amino-1-phenylethyl)phosphonate". Org. Synth. 94: 313–331. doi:10.15227/orgsyn.094.0313.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.