Hydrophosphination
Hydrophosphination is the insertion of a carbon-carbon multiple bond into a phosphorus-hydrogen bond forming a new phosphorus-carbon bond. Like other hydrofunctionalizations, the rate and regiochemistry of the insertion reaction is influenced by the catalyst. Catalysts take many forms, but most prevalent are bases and free-radical initiators.[1]
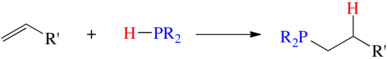
Acid-base routes
The usual application of hydrophosphination involves reactions of phosphine (PH3). Typically base-catalysis allows addition of Michael acceptors such as acrylonitrile to give tris(cyanoethyl)phosphine:[1]
- PH3 + 3 CH2=CHZ → P(CH2CH2Z)3 (Z = NO2, CN, C(O)NH2)
Acid catalysis is applicable to hydrophosphination with alkenes that form stable carbocations. These alkenes include isobutylene and many analogues:[1]
- PH3 + R2C=CH2 → R2(CH3)CPH2 (R = Me, alkyl, etc)
Bases catalyze the addition of secondary phosphines to vinyldiphenylphosphine:[2]
- HPR2 + CH2=CHPR'2 → R2PCH2CH2PR'2
Free-radical methods
Many hydrophosphination reactions are initiated by free-radicals. AIBN and peroxides are typical initiators, as well as Ultraviolet irradiation. In this way, the commercially important tributylphosphine and trioctylphosphine are prepared in good yields from 1-butene and 1-octene, respectively.[1]
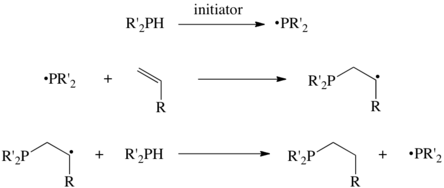
The reactions proceed by abstraction of an H atom the phosphine precursor, producing the phosphino radical, a seven electron species. This radical then adds to the alkene, and subsequent H-atom transfer completes the cycle.[3] Some highly efficient hydrophosphinations appear not to proceed via radicals, but alternative explanations are lacking.[4]
Metal-catalyzed reactions
Metal-catalyzed hydrophosphinations are not widely used, although they have been extensively researched. Studies mainly focus on secondary and primary organophosphines (R2PH and RPH2, respectively). These substrates bind to metals, and the resulting adducts insert alkenes and alkynes into the P-H bonds via diverse mechanisms.[5][6][7]
Early transition metal and lanthanide catalysts
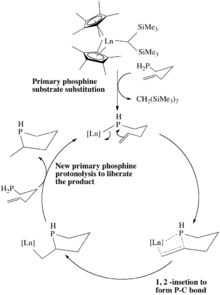
Metal complexes of d0 configurations are effective catalysts for hydrophosphinations of simple alkenes and alkynes.[8][9] Intramolecular reactions are facile, e.g. starting with α,ω-pentenylphosphine. The primary phosphine undergoes a σ-bond metathesis with the bis(trimethylsilyl)methylene ligand forming the lanthanide-phosphido complex. Subsequently the pendant terminal alkene or alkyne inserts into the Ln-P bond. Finally, protonolysis of the Ln-C bond with the starting primary phosphine releases the new phosphine and regenerates the catalyst. Given that the metal is electron-poor, the M-C bond is sufficiently enough to be protonolyzed by the substrate primary phosphine.
Most metal catalyzed hydrophosphinations proceed via metal phosphido intermediates. Some however proceed by metal-phosphinidene intermediates, i.e. species with M=PR double bonds. One such example is the Ti-catalyzed hydrophosphination of diphenylacetylene with phenylphosphine.[10] This system involves a cationic catalyst precursor that is stabilized by the bulky 2,4,6-tri(isopropyl)phenyl- substituent on the phosphinidene and the close ionic association of methyltris(pentafluorophenyl)borate. This precursor undergoes exchange with phenylphosphine to give the titanium-phenylphosphinidene complex, which is the catalyst. The Ti=PPh species undergoes a [2+2] cycloaddition with diphenylacetylene to make the corresponding metallacyclobutene. The substrate, phenylphosphine, protonolyzes the Ti-C bond and after a proton shift regenerates the catalyst and releases the new phosphine.
Titanium-catalyzed 1,4-hydrophosphination of 1,3-dienes with diphenylphosphine has been demonstrated.[11] It is a rare example of a d2 catalyst. In the first step, the Ti(II) precursor inserted in the P-H bond ofdiphenylphosphine (Ph2PH).
Late transition metal catalysts
Late transition metal hydrophosphination catalysts, i.e. those reliant on the nickel-triad and neighboring elements, generally require alkenes and alkynes with electron withdrawing substituents. A strong base is required as a cocatalyst.[6]
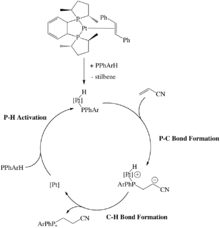
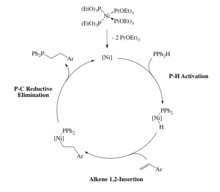
Some late metal hydrophosphination catalysts proceed via oxidative addition of a P-H bond. For example, a Pt(0) catalyst undergoes oxidative addition of a secondary phosphine to form the corresponding hydrido Pt(II) phosphido complex. These systems catalyze hydrophosphination of acrylonitrile, although this reaction can be achieved without metal catalysts. The key P-C bond-forming step occurs through an outer-sphere, Michael-type addition.[6]
The usual mechanism for hydrophosphination for late metal catalysts involves insertion of the alkene into the metal-phosphorus bond. Insertion into the metal-hydrogen bond is also possible. The product phosphine is produced through reductive elimination of a P-C bond rather than a P-H bond in Glueck's system.[12][13] The Ni(0) catalyst involves oxidation addition of a P-H bond to the metal, followed by insertion of the alkene into the M-H bond.
Hydrophosphorylation and related reactions
Utilizing phosphorus(V) precursors hydrophosphorylation entails the insertion of alkenes and alkynes into the P-H bonds of secondary phosphine oxides:[14]
- R2P(O)H + CH2=CHR → R2P(O)CH2CH2R
The reaction can be effected both using metal catalysts or free-radical initiators.
Further reading
- Motta, A.; Fragalà, I. L.; Marks, T. J. (2005). "Energetics and Mechanism of Organolanthanide-Mediated Phosphinoalkene Hydrophosphination/Cyclization. A Density Functional Theory Analysis". Organometallics. 24 (21): 4995. doi:10.1021/om050570d.
- Douglass, M. R.; Stern, C. L.; Marks, T. J. (2001). "Intramolecular Hydrophosphination/Cyclization of Phosphinoalkenes and Phosphinoalkynes Catalyzed by Organolanthanides: Scope, Selectivity, and Mechanism". Journal of the American Chemical Society. 123 (42): 10221. doi:10.1021/ja010811i.
- Douglass, M. R.; Marks, T. J. (2000). "Organolanthanide-Catalyzed Intramolecular Hydrophosphination/Cyclization of Phosphinoalkenes and Phosphinoalkynes". Journal of the American Chemical Society. 122 (8): 1824. doi:10.1021/ja993633q.
- Douglass, M. R.; Ogasawara, M.; Hong, S.; Metz, M. V.; Marks, T. J. (2002). ""Widening the Roof": Synthesis and Characterization of New ChiralC1-Symmetric Octahydrofluorenyl Organolanthanide Catalysts and Their Implementation in the Stereoselective Cyclizations of Aminoalkenes and Phosphinoalkenes". Organometallics. 21 (2): 283. doi:10.1021/om0104013.
- Kawaoka, A. M.; Douglass, M. R.; Marks, T. J. (2003). "Homoleptic Lanthanide Alkyl and Amide Precatalysts Efficiently Mediate Intramolecular Hydrophosphination/Cyclization. Observations on Scope and Mechanism". Organometallics. 22 (23): 4630. doi:10.1021/om030439a.
- Scriban, C.; Glueck, D. S.; Zakharov, L. N.; Kassel, W. S.; Dipasquale, A. G.; Golen, J. A.; Rheingold, A. L. (2006). "P−C and C−C Bond Formation by Michael Addition in Platinum-Catalyzed Hydrophosphination and in the Stoichiometric Reactions of Platinum Phosphido Complexes with Activated Alkenes". Organometallics. 25 (24): 5757. doi:10.1021/om060631n.
- Scriban, C.; Kovacik, I.; Glueck, D. S. (2005). "A Protic Additive Suppresses Formation of Byproducts in Platinum-Catalyzed Hydrophosphination of Activated Olefins. Evidence for P−C and C−C Bond Formation by Michael Addition". Organometallics. 24 (21): 4871. doi:10.1021/om050433g.
- Wicht, D. K.; Kourkine, I. V.; Lew, B. M.; Nthenge, J. M.; Glueck, D. S. (1997). "Platinum-Catalyzed Acrylonitrile Hydrophosphination via Olefin Insertion into a Pt−P Bond". Journal of the American Chemical Society. 119 (21): 5039. doi:10.1021/ja970355r.
- Kovacik, I.; Wicht, D. K.; Grewal, N. S.; Glueck, D. S.; Incarvito, C. D.; Guzei, I. A.; Rheingold, A. L. (2000). "Pt(Me-Duphos)-Catalyzed Asymmetric Hydrophosphination of Activated Olefins: Enantioselective Synthesis of Chiral Phosphines". Organometallics. 19 (6): 950. doi:10.1021/om990882e.
- Pringle, P. G.; Smith, M. B. (1990). "Platinum(0)-catalysed hydrophosphination of acrylonitrile". Journal of the Chemical Society, Chemical Communications (23): 1701. doi:10.1039/C39900001701.
- Sadow, A. D.; Togni, A. (2005). "Enantioselective Addition of Secondary Phosphines to Methacrylonitrile: Catalysis and Mechanism". Journal of the American Chemical Society. 127 (48): 17012. doi:10.1021/ja0555163.
- Huang, Y.; Pullarkat, S. A.; Li, Y.; Leung, P. H. (2010). "Palladium(ii)-catalyzed asymmetric hydrophosphination of enones: Efficient access to chiral tertiary phosphines". Chemical Communications. 46 (37): 6950–2. doi:10.1039/C0CC00925C. PMID 20730193.
- Xu, C.; Jun Hao Kennard, G.; Hennersdorf, F.; Li, Y.; Pullarkat, S. A.; Leung, P. H. (2012). "Chiral Phosphapalladacycles as Efficient Catalysts for the Asymmetric Hydrophosphination of Substituted Methylidenemalonate Esters: Direct Access to Functionalized Tertiary Chiral Phosphines". Organometallics. 31 (8): 3022. doi:10.1021/om201115n.
- Huang, Y.; Pullarkat, S. A.; Teong, S.; Chew, R. J.; Li, Y.; Leung, P. H. (2012). "Palladacycle-Catalyzed Asymmetric Intermolecular Construction of Chiral Tertiary P-Heterocycles by Stepwise Addition of H–P–H Bonds to Bis(enones)". Organometallics. 31 (13): 4871. doi:10.1021/om300405h.
- Huang, Y.; Pullarkat, S. A.; Li, Y.; Leung, P. H. (2012). "Palladacycle-Catalyzed Asymmetric Hydrophosphination of Enones for Synthesis of C*- and P*-Chiral Tertiary Phosphines". Inorganic Chemistry. 51 (4): 2533–40. doi:10.1021/ic202472f. PMID 22289417.
- Huang, Y.; Chew, R. J.; Li, Y.; Pullarkat, S. A.; Leung, P. H. (2011). "Direct Synthesis of Chiral Tertiary Diphosphinesvia Pd(II)-Catalyzed Asymmetric Hydrophosphination of Dienones". Organic Letters. 13 (21): 5862–5. doi:10.1021/ol202480r. PMID 21985055.
- Derrah, E. J.; Pantazis, D. A.; McDonald, R.; Rosenberg, L. (2007). "A Highly Reactive Ruthenium Phosphido Complex Exhibiting Ru−P π-Bonding". Organometallics. 26 (6): 1473. doi:10.1021/om0700056.
- Derrah, E. J.; Pantazis, D. A.; McDonald, R.; Rosenberg, L. (2010). "Concerted [2+2] Cycloaddition of Alkenes to a Ruthenium-Phosphorus Double Bond". Angewandte Chemie International Edition. 49 (19): 3367. doi:10.1002/anie.201000356. PMID 20358572.
- Derrah, E. J.; McDonald, R.; Rosenberg, L. (2010). "The [2+2] cycloaddition of alkynes at a Ru–P π-bond". Chemical Communications. 46 (25): 4592. doi:10.1039/C002765K. PMID 20458386.
- Gibson, G. L.; Morrow, K. M. E.; McDonald, R.; Rosenberg, L. (2011). "Diastereoselective synthesis of a "chiral-at-Ru" secondary phosphine complex". Inorganica Chimica Acta. 369: 133–139. doi:10.1016/j.ica.2010.12.058.
References
- Trofimov, Boris A.; Arbuzova, Svetlana N.; Gusarova, Nina K. (1999). "Phosphine in the synthesis of organophosphorus compounds". Russian Chemical Reviews. 68: 215–227. Bibcode:1999RuCRv..68..215T. doi:10.1070/RC1999v068n03ABEH000464.
- King, R. Bruce (1972). "Poly(tertiary Phosphines) and Their Metal Complexes". Accounts of Chemical Research. 5: 177–185. doi:10.1021/ar50053a003.
- Quin, L. D. A Guide to Organophosphorus Chemistry; John Wiley & Sons: New York, 2000; pp 28-29.
- Alonso, Francisco; Moglie, Yanina; Radivoy, Gabriel; Yus, Miguel (2012). "Solvent- and catalyst-free regioselective hydrophosphanation of alkenes". Green Chemistry. 14: 2699. doi:10.1039/c2gc35898k.
- Greenberg, S.; Stephan, D. W. (2008). "Stoichiometric and catalytic activation of P–H and P–P bonds". Chemical Society Reviews. 37 (8): 1482. doi:10.1039/B612306F. PMID 18648674.
- Glueck, David S. (2010). "Recent Advances in Metal-Catalyzed C–P Bond Formation". C-X Bond Formation. Topics in Organometallic Chemistry. 31. pp. 65–100. doi:10.1007/978-3-642-12073-2_4. ISBN 978-3-642-12072-5.
- Rosenberg, L. R. ACS Catal. 2013, 3, 2845.Rosenberg, L. (2013). "Mechanisms of Metal-Catalyzed Hydrophosphination of Alkenes and Alkynes". ACS Catalysis. 3 (12): 2845–2855. doi:10.1021/cs400685c.
- Bange, Christine A.; Waterman, Rory (2016). "Challenges in Catalytic Hydrophosphination". Chemistry - A European Journal. 22 (36): 12598–12605. doi:10.1002/chem.201602749. PMID 27405918.
- Trifonov, A. A.; Basalov, I. V.; Kissel, A. A. (2016). "Use of organolanthanides in the catalytic intermolecular hydrophosphination and hydroamination of multiple C–C bonds". Dalton Transactions. 45 (48): 19172–19193. doi:10.1039/C6DT03913H. PMID 27891536.
- Zhao, G.; Basuli, F.; Kilgore, U. J.; Fan, H.; Aneetha, H.; Huffman, J. C.; Wu, G.; Mindiola, D. J. (2006). "Neutral and Zwitterionic Low-Coordinate Titanium Complexes Bearing the Terminal Phosphinidene Functionality. Structural, Spectroscopic, Theoretical, and Catalytic Studies Addressing the Ti−P Multiple Bond". Journal of the American Chemical Society. 128 (41): 13575–85. doi:10.1021/ja064853o. PMID 17031972.
- Perrier, A.; Comte, V.; Moïse, C.; Le Gendre, P. (2010). "First Titanium-Catalyzed 1,4-Hydrophosphination of 1,3-Dienes". Chemistry – A European Journal. 16 (1): 64–67. doi:10.1002/chem.200901863. PMID 19918817.
- Shulyupin, M. O.; Kazankova, M. A.; Beletskaya, I. P. (2002). "Catalytic Hydrophosphination of Styrenes". Organic Letters. 4 (5): 761–3. doi:10.1021/ol017238s. PMID 11869121.
- Kazankova, M. A.; Shulyupin, M. O.; Borisenko, A. A.; Beletskaya, I. P. (2002). "Synthesis of Alkyl(diphenyl)phosphines by Hydrophosphination of Vinylarenes Catalyzed by Transition Metal Complexes". Russian Journal of Organic Chemistry. 38 (10): 1479. doi:10.1023/A:1022552404812.
- Han, Li-Biao; Ono, Yutaka; Xu, Qing; Shimada, Shigeru (2010). "Highly Selective Markovnikov Addition of Hypervalent H-Spirophosphoranes to Alkynes Mediated by Palladium Acetate: Generality and Mechanism". 83: 1086–1099. doi:10.1246/bcsj.20100141. Cite journal requires
|journal=(help)