Hydrofunctionalization
A hydrofunctionalization reaction is the addition of hydrogen and another univalent fragment (X) across a carbon-carbon or carbon-heteroatom multiple bond.[1] Often, the term hydrofunctionalization without modifier refers specifically to the use of the covalent hydride (H-X) as the source of hydrogen and X for this transformation. If other reagents are used to achieve the net addition of hydrogen and X across a multiple bond, the process may be referred to as a formal hydrofunctionalization.
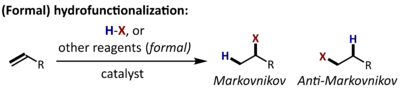
For terminal olefins (or acetylenes), the regioselectivity of the process can be described as Markovnikov (addition of X at the substituted end) or anti-Markovnikov (addition of X at the unsubstituted end). Catalysts are frequently employed to control the chemo-, regio-, and stereoselectivity of hydrofunctionalization reactions.
Examples
Some of the better known classes of hydrofunctionalization reactions include the following:
- Hydroboration
- Hydrosilylation
- Hydrometalation (including both transition or main group metal hydrides)
- Hydroamination
- (Olefin) hydration (addition of H2O across a double bond)
- Hydroalkoxylation (also known as hydroetherification)
- Hydrohalogenation
- Hydrovinylation (including hydroarylation and olefin dimerization and others)
- Hydroacylation
- Hydroformylation (refers specifically to the addition of CHO and H using H2 and CO as reagents, also known as the oxo process)
References
- Hydrofunctionalization - Springer. Topics in Organometallic Chemistry. 43. 2013. doi:10.1007/978-3-642-33735-2. ISBN 978-3-642-33734-5.