Human T-lymphotropic virus
The human T-lymphotropic virus, human T-cell lymphotropic virus, or human T-cell leukemia-lymphoma virus (HTLV) family of viruses are a group of human retroviruses that are known to cause a type of cancer called adult T-cell leukemia/lymphoma and a demyelinating disease called HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP). The HTLVs belong to a larger group of primate T-lymphotropic viruses (PTLVs). Members of this family that infect humans are called HTLVs, and the ones that infect Old World monkeys are called Simian T-lymphotropic viruses (STLVs). To date, four types of HTLVs (HTLV-1, HTLV-2, HTLV-3, and HTLV-4) and four types of STLVs (STLV-1, STLV-2, STLV-3, and STLV-5) have been identified. HTLV types HTLV-1 and HTLV-2 viruses are the first retroviruses which were discovered. Both belong to the oncovirus subfamily of retroviruses and can transform human lymphocytes so that they are self-sustaining in vitro.[1] The HTLVs are believed to originate from interspecies transmission of STLVs. The HTLV-1 genome is diploid, composed of two copies of a single-stranded RNA virus whose genome is copied into a double-stranded DNA form that integrates into the host cell genome, at which point the virus is referred to as a provirus. A closely related virus is bovine leukemia virus BLV. The original name for HIV, the virus that causes AIDS, was HTLV-3.
| Human T-lymphotropic virus | |
|---|---|
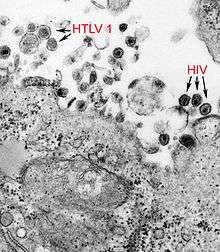 | |
| a micrograph showing both Human T-lymphotropic virus 1 and HIV | |
| Scientific classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Pararnavirae |
| Phylum: | Artverviricota |
| Class: | Revtraviricetes |
| Order: | Ortervirales |
| Family: | Retroviridae |
| Subfamily: | Orthoretrovirinae |
| Genus: | Deltaretrovirus |
| Groups included | |
| |
| Cladistically included but traditionally excluded taxa | |
| |
HTLV-1
HTLV-1 is an abbreviation for human T-cell lymphotropic virus type 1, also called human T-cell leukemia type 1, a virus that has been implicated in several kinds of diseases, including tropical spastic paraparesis, and as a virus cancer link for leukemia (see adult T-cell leukemia/lymphoma). HTLV-1 has six reported subtypes (subtypes A to F). The great majority of infections are caused by the cosmopolitan subtype A.[2] HTLV was discovered by Robert Gallo and colleagues in 1980.[3] Between 1 in 20 and 1 in 25 infected people are thought to develop cancer as a result of the virus.[4] HTLV-1 infection is thought to spread only through dividing cells since reverse transcriptase generates proviral DNA from genomic viral RNA, and the provirus is integrated into the host genome by viral integrase after transmission. Therefore, the quantification of provirus reflects the number of HTLV-1-infected cells. So, an increase in numbers of HTLV-1-infected cells using cell division, by actions of accessory viral genes, especially Tax, may provide an enhancement of infectivity. Tax expression induces proliferation, inhibits the apoptosis of HTLV-1-infected cells and, conversely, evokes the host immune response, including cytotoxic T cells, to kill virus-infected cells.[5]
_Mycosis_Fungoides.jpg)
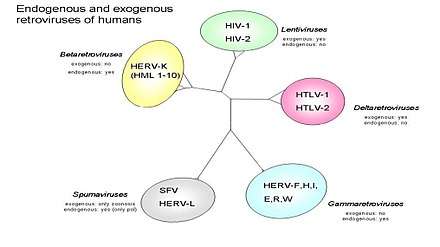
HTLV-2
A virus closely related to HTLV-1, also discovered by Robert Gallo and colleagues. The family of Human T-lymphotropic virus (Figure 2) can be further categorized into four sub types. The figure also divides the retroviruses into exogenous and endogenous. Retroviruses can exist as two different forms: endogenous which consist of normal genetic components and exogenous which are horizontally transferred genetic components that are usually infectious agents that cause disease i.e. HIV. In (Figure 3) open reading frames (ORF) are shown which can if translated can predict which genes will be present and this can help to better understand human retroviruses. Of the four subtypes, HTLV-2 may be linked to Cutaneous T-cell lymphoma (CTCL).[7] In one study involving cultured lymphocytes from patients with mycosis fungoides (Figure 1), PCR amplification showed gene sequences of HTLV-II.[7] This finding may suggest a possible correlation with HTLV-2 and CTCL. Further research and studies must be conducted to show a positive relationship.
HTLV-3 and HTLV-4
HTLV-3 and HTLV-4 have been used to describe recently characterized viruses.[9][10][11] These viruses were discovered in 2005 in rural Cameroon, and were, it is presumed, transmitted from monkeys to hunters of monkeys through bites and scratches.
- HTLV-3 is similar to STLV-3 (Simian T-lymphotropic virus 3). Multiple strains have been identified.[13] It expresses gag, pol, and env, among other proteins.[14]
- HTLV-4 is apparently substantially identical to STLV-4 hosted in gorillas.
It is not yet known how much further transmission has occurred among humans, or whether the viruses can cause disease.
The use of these names can cause some confusion, because the name HTLV-3 was one of the names for HIV in early AIDS literature, but has since fallen out of use.[15] The name HTLV-4 has also been used to describe HIV-2.[16] A large Canadian study documented this confusion among healthcare workers, where >90% of HTLV tests ordered by physicians were actually intended to be HIV tests.[17]

Transmission
HTLV-1 and HTLV-2 can be transmitted sexually,[19][20] by blood to blood contact (e.g. by blood transfusion or sharing needles when using drugs)[21][22] and via breast feeding.[23]
Epidemiology
Two HTLVs are well established. HTLV-1 and HTLV-2 are both involved in actively spreading epidemics, affecting 15-20 million people worldwide.[24]
HTLV-1 is the most clinically significant of the two: at least 500,000 of the individuals infected with HTLV-1 eventually develop an often rapidly fatal leukemia, while others will develop a debilitative myelopathy, and yet others will experience uveitis, infectious dermatitis, or another inflammatory disorder. HTLV-2 is associated with milder neurologic disorders and chronic pulmonary infections. In the United States, HTLV-1/2 seroprevalence rates among volunteer blood donors average 0.016 percent.
No specific illnesses have yet been associated with HTLV-3 and HTLV-4.
Vaccination and treatments
While there is no present licensed vaccine, there are many factors which make a vaccine against HTLV-1 feasible. The virus displays relatively low antigenic variability, natural immunity does occur in humans, and experimental vaccination using envelope antigens has been shown to be successful in animal models. Plasmid DNA vaccines elicit potent and protective immune responses in numerous small-animal models of infectious diseases. However, their immunogenicity in primates appears less potent. In the past two decades a large initiative has been put forth to understand the biological and pathogenic properties of the human T-cell lymphotropic virus type 1 (HTLV-1); this has ultimately led to the development of various experimental vaccination and therapeutic strategies to combat HTLV-1 infection. These strategies include the development of envelope glycoprotein derived B-cell epitopes for the induction of neutralizing antibodies, as well as a strategy to generate a multivalent cytotoxic T-lymphocyte (CTL) response against the HTLV-1 Tax antigen. A vaccine candidate that can elicit or boost anti-gp46 neutralizing antibody response may have a potential for prevention and therapy against HTLV-1 infection.[25]
Potential treatments include prosultiamine, a vitamin B-1 derivative, which has been shown to reduce viral load and symptoms;[26] azacytidine, an anti-metabolite, which has been credited with the cure of a patient in Greece;[27] tenofovir disoproxil (TDF), a reverse-transcriptase inhibitor used for HIV; cepharanthine, an alkaloid from stephania cepharantha hayata;[28] and phosphonated carbocyclic 2'-oxa-3'aza nucleosides (PCOANs).[29] A newer formulation of TDF, called tenofovir alafenamide (TAF), also has promise as a treatment with less toxicity.
References
- "Recommendations for counseling persons infected with human T-lymphotrophic virus, types I and II. Centers for Disease Control and Prevention and U.S. Public Health Service Working Group". MMWR Recomm Rep. 42 (RR-9): 1–13. 1993. PMID 8393133. Archived from the original on 2017-08-29. Retrieved 2017-09-09.
- Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC, Carneiro-Proietti AB (2010). "Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases". Clin. Microbiol. Rev. 23 (3): 577–89. doi:10.1128/CMR.00063-09. PMC 2901658. PMID 20610824.
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC (1980). "Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma". Proc. Natl. Acad. Sci. U.S.A. 77 (12): 7415–9. Bibcode:1980PNAS...77.7415P. doi:10.1073/pnas.77.12.7415. PMC 350514. PMID 6261256.
- WELSH, JAMES S. (January 2011). "Contagious Cancer". The Oncologist. 16 (1): 1–4. doi:10.1634/theoncologist.2010-0301. PMC 3228048. PMID 21212437.
- Taylor GP, Matsuoka M (2005). "Natural history of adult T-cell leukemia/lymphoma and approaches to therapy". Oncogene. 24 (39): 6047–57. doi:10.1038/sj.onc.1208979. PMID 16155611.
- Norman Purvis, Walker (1905). An introduction to dermatology (3rd ed.). William Wood and Company.
- Mirvish, Ezra D.; Pomerantz, Rebecca G.; Geskin, Larisa J. (2011). "Infectious agents in cutaneous T-cell lymphoma". Journal of the American Academy of Dermatology. 64 (2): 423–431. doi:10.1016/j.jaad.2009.11.692. PMC 3954537. PMID 20692726.
- van der Kuyl, Antoinette C. (2012-01-16). "HIV infection and HERV expression: a review". Retrovirology. 9: 6. doi:10.1186/1742-4690-9-6. ISSN 1742-4690. PMC 3311604. PMID 22248111.
- Mahieux, R; Gessain, A (2005). "Les nouveaux rétrovirus humains HTLV-3 et HTLV-4" [New human retroviruses: HTLV-3 and HTLV-4] (PDF). Médecine Tropicale (in French). 65 (6): 525–8. PMID 16555510. Archived (PDF) from the original on 2011-07-15. Retrieved 2010-12-10.
- Calattini, S.; Chevalier, S. A.; Duprez, R.; Afonso, P.; Froment, A.; Gessain, A.; Mahieux, R. (2006). "Human T-Cell Lymphotropic Virus Type 3: Complete Nucleotide Sequence and Characterization of the Human Tax3 Protein". Journal of Virology. 80 (19): 9876–88. doi:10.1128/JVI.00799-06. PMC 1617244. PMID 16973592.
- Mahieux, R.; Gessain, Antoine (2009). "The human HTLV-3 and HTLV-4 retroviruses: New members of the HTLV family". Pathologie Biologie. 57 (2): 161–6. doi:10.1016/j.patbio.2008.02.015. PMID 18456423.
- Calattini, Sara; Betsem, Edouard; Bassot, Sylviane; Chevalier, SéBastien Alain; Mahieux, Renaud; Froment, Alain; Gessain, Antoine (2009). "New Strain of Human T Lymphotropic Virus (HTLV) Type 3 in a Pygmy from Cameroon with Peculiar HTLV Serologic Results" (PDF). The Journal of Infectious Diseases. 199 (4): 561–4. doi:10.1086/596206. PMID 19099485. Archived (PDF) from the original on 2019-09-01. Retrieved 2019-09-01.
- Chevalier, S. A.; Ko, N. L.; Calattini, S.; Mallet, A.; Prevost, M.-C.; Kehn, K.; Brady, J. N.; Kashanchi, F.; et al. (2008). "Construction and Characterization of a Human T-Cell Lymphotropic Virus Type 3 Infectious Molecular Clone". Journal of Virology. 82 (13): 6747–52. doi:10.1128/JVI.00247-08. PMC 2447071. PMID 18417569.
- Human+T-Lymphotropic+Virus+Type+III at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human+T+Lymphotropic+Virus+Type+IV at the US National Library of Medicine Medical Subject Headings (MeSH)
- Siemieniuk, Reed; Fonseca, Kevin; Gill M. John (November 2012). "Using Root Cause Analysis and Form Redesign to Reduce Incorrect Ordering of HIV Tests". The Joint Commission Journal on Quality and Patient Safety. 11. 38 (11): 506–512. doi:10.1016/S1553-7250(12)38067-7. PMID 23173397.
- Armbruester, V; Sauter, M; Krautkraemer, E; Meese, E; Kleiman, A; Best, B; et al. "A novel gene from the human endogenous retrovirus K expressed in transformed cells". Clin Cancer Res. 2002 (8): 1800–1807.
- Rodriguez, Evelyn M.; de Moya, E. Antonio; Guerrero, Ernesto; Monterroso, Edgar R.; Quinn, Thomas C.; Puello, Elizardo; de Quiñones, Margarita Rosado; Thorington, Bruce; et al. (1993). "HIV-1 and HTLV-I in sexually transmitted disease clinics in the Dominican Republic". Journal of Acquired Immune Deficiency Syndromes. 6 (3): 313–8. PMID 8450407. Archived from the original on 2012-10-06. Retrieved 2010-12-10.
- Roucoux, Diana F.; Wang, Baoguang; Smith, Donna; Nass, Catharie C.; Smith, James; Hutching, Sheila T.; Newman, Bruce; Lee, Tzong-Hae; et al. (2005). "A Prospective Study of Sexual Transmission of Human T Lymphotropic Virus (HTLV)-I and HTLV-II". The Journal of Infectious Diseases. 191 (9): 1490–7. doi:10.1086/429410. PMID 15809908.
- "Archived copy". Archived from the original on 2015-11-02. Retrieved 2015-11-04.CS1 maint: archived copy as title (link)
- "Archived copy". Archived from the original on 2015-11-20. Retrieved 2015-11-04.CS1 maint: archived copy as title (link)
- Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell ML (2007). "Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study". Lancet. 369 (9567): 1107–16. doi:10.1016/S0140-6736(07)60283-9. PMID 17398310.
- de Thé, G.; Kazanji, M. (1996). "An HTLV-I/II vaccine: from animal models to clinical trials?". Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 13 Suppl 1: S191–198. doi:10.1097/00042560-199600001-00029. ISSN 1077-9450. PMID 8797723.
- Tanaka Y, Takahashi Y, Kodama A, Tanaka R, Saito M (2014). "Neutralizing antibodies against human T cell leukemia virus type-I (HTLV-1) eradicate HTLV-1 in combination with autologous peripheral blood mononuclear cells via antibody-dependent cellular cytotoxicity while preventing new infection" (PDF). Retrovirology. 11 (Suppl 1): O39. doi:10.1186/1742-4690-11-s1-o39. Archived (PDF) from the original on 2015-09-10. Retrieved 2014-03-05.
- "Nervous System Disease: A New Outlet for an Old Drug?". Archived from the original on 2018-01-18. Retrieved 2018-03-09.
- Diamantopoulos PT, Michael M, Benopoulou O, Bazanis E, Tzeletas G, Meletis J, Vayopoulos G, Viniou NA (2012). "Antiretroviral activity of 5-azacytidine during treatment of a HTLV-1 positive myelodysplastic syndrome with autoimmune manifestations". Virol. J. 9: 1. doi:10.1186/1743-422X-9-1. PMC 3305386. PMID 22214262.
- Toyama, M; Hamasaki, T; Uto, T; Aoyama, H; Okamoto, M; Hashmoto, Y; Baba, M (2012). "Synergistic inhibition of HTLV-1-infected cell proliferation by combination of cepharanthine and a tetramethylnaphthalene derivative". Anticancer Research. 32 (7): 2639–45. PMID 22753721.
- MacChi, B.; Balestrieri, E.; Ascolani, A.; Hilburn, S.; Martin, F.; Mastino, A.; Taylor, G. P. (2011). "Susceptibility of Primary HTLV-1 Isolates from Patients with HTLV-1-Associated Myelopathy to Reverse Transcriptase Inhibitors". Viruses. 3 (12): 469–483. doi:10.3390/v3050469. PMC 3185762. PMID 21994743.
External links
- human T-cell lymphotrophic virus at eMedicine
- International Retrovirology Association
- Human+T-lymphotropic+virus+1 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human+T-lymphotropic+virus+2 at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Human T-lymphotropic virus 1". NCBI Taxonomy Browser. 11908.
- "Human T-lymphotropic virus 2". NCBI Taxonomy Browser. 11909.
- "Human T-lymphotropic virus 3". NCBI Taxonomy Browser. 28332.
- "Human T-lymphotropic virus 4". NCBI Taxonomy Browser. 318279.
- "Untyped Human T-lymphotropic virus". NCBI Taxonomy Browser. 318275.