Hormesis
Hormesis is any process in a cell or organism that exhibits a biphasic response to exposure to increasing amounts of a substance or condition.[1] Within the hormetic zone, there is generally a favorable biological response to low exposures to toxins and other stressors. Hormesis comes from Greek hórmēsis "rapid motion, eagerness", itself from ancient Greek hormáein "to set in motion, impel, urge on". Hormetics is the term proposed for the study and science of hormesis.
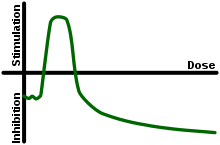
In toxicology, hormesis is a dose response phenomenon characterized by a low dose stimulation, high dose inhibition, resulting in either a J-shaped or an inverted U-shaped dose response.[1] Such environmental factors that would seem to produce positive responses have also been termed "eustress". The hormesis model of dose response is vigorously debated.[2] The notion that hormesis is important for chemical risk regulations is not widely accepted.[3]
The biochemical mechanisms by which hormesis works remain under laboratory research and are not well understood.[1]
History
German pharmacologist Hugo Schulz first described such a phenomenon in 1888 following his own observations that the growth of yeast could be stimulated by small doses of poisons. This was coupled with the work of German physician Rudolph Arndt, who studied animals given low doses of drugs, eventually giving rise to the Arndt-Schulz rule.[2] Arndt's advocacy of homeopathy contributed to the rule's diminished credibility in the 1920s and 1930s.[2] The term "hormesis" was coined and used for the first time in a scientific paper by Chester M. Southam and J. Ehrlich in 1943 in the journal: Phytopathology, volume 33, pp. 517–541. Recently, Edward Calabrese has revived the concept of hormesis.[4][5]
Examples
Physical exercise
Individuals with low levels of physical activity are at risk for high levels of oxidative stress, as are individuals engaged in highly intensive exercise programs; however individuals engaged in moderately intensive, regular exercise experience lower levels of oxidative stress. High levels of oxidative stress have been linked by some with the increased incidence of a variety of diseases.[6]
It has been claimed that this relationship, characterized by positive effects at an intermediate dose of the stressor (exercise), is characteristic of hormesis.[6] However, it is important to point out that there is evidence that the oxidative stress associated with intensive exercise may have long-term health benefits. This would imply that oxidative stress, itself, provides an example of hormesis (see section on Mitochondrial hormesis), but physical exercise does not.[7]
Alcohol
Alcohol is believed to be hormetic in preventing heart disease and stroke,[8] although the benefits of light drinking may have been exaggerated.[9][10]
In 2012, researchers at UCLA found that tiny amounts (1 mM, or 0.005%) of ethanol doubled the lifespan of Caenorhabditis elegans, a round worm frequently used in biological studies, that were starved of other nutrients. Higher doses of 0.4% provided no longevity benefit.[11] However, worms exposed to 0.005% did not develop normally (their development was arrested). The authors argue that the worms were using ethanol as an alternative energy source in the absence of other nutrition, or had initiated a stress response. They did not test the effect of ethanol on worms fed a normal diet.
As light alcohol consumption increases cancer risk, it doesn't have a hormetic zone from the point of view of cancer.
Methylmercury and mallard eggs
In 2010, a paper published in the journal Environmental Toxicology & Chemistry showed that low doses of methylmercury, a potent neurotoxic pollutant, improved the hatching rate of mallard eggs.[12] The author of the study, Gary Heinz, who led the study for the U.S. Geological Survey at the Patuxent Wildlife Research Center in Beltsville, Md., stated that other explanations are possible. For instance, it is possible that the flock he studied might have harbored some low, subclinical infection and that mercury, well known to be antimicrobial, might have killed the infection that otherwise hurt reproduction in the untreated birds.[12]
Effects in aging
One of the areas where the concept of hormesis has been explored extensively with respect to its applicability is aging.[13][14] Since the basic survival capacity of any biological system depends on its homeostatic ability, biogerontologists proposed that exposing cells and organisms to mild stress should result in the adaptive or hormetic response with various biological benefits. This idea has now gathered a large body of supportive evidence showing that repetitive mild stress exposure has anti-aging effects.[15][16] Exercise is a paradigm for hormesis in this respect.[16] Some of the mild stresses used for such studies on the application of hormesis in aging research and interventions are heat shock, irradiation, prooxidants, hypergravity, and food restriction.[15][16][17] Some other natural and synthetic molecules, such as celastrols from medicinal herbs and curcumin from the spice turmeric have also been found to have hormetic beneficial effects.[18] Such compounds which bring about their health beneficial effects by stimulating or by modulating stress response pathways in cells have been termed "hormetins".[15] Hormetic interventions have also been proposed at the clinical level,[19] with a variety of stimuli, challenges and stressful actions, that aim to increase the dynamical complexity of the biological systems in humans.[20]
Mitochondria
Mitochondria are sometimes described as "cellular power plants" because they generate most of the cell's supply of adenosine triphosphate (ATP), a source of chemical energy. Reactive oxygen species (ROS) have been regarded as unwanted by-products of oxidative phosphorylation in mitochondria by the proponents of the free-radical theory of aging promoted by Denham Harman. The free-radical theory suggests that the use of compounds which inactivate ROS, such as antioxidants, would lead to a reduction of oxidative stress and thereby produce an increase in lifespan.
ROS may perform an essential and potentially lifespan-promoting role as redox signaling molecules which transduce signals from the mitochondrial compartment to other compartments of the cell.[21] Increased formation of ROS within the mitochondria may cause an adaptive reaction which produces increased stress resistance and a long-term reduction of oxidative stress. This kind of reverse effect of the response to ROS stress has been named mitochondrial hormesis or mitohormesis and is hypothesized to be responsible for the respective lifespan-extending and health-promoting capabilities of glucose restriction and physical exercise.[21]
Whether this concept applies to humans remains to be shown, although recent epidemiological findings support the process of mitohormesis, and even suggest that some antioxidant supplements may increase disease prevalence in humans.[22]
Ionizing radiation
Hormesis has been observed in a number of cases in humans and animals exposed to chronic low doses of ionizing radiation. A-bomb survivors who received high doses exhibited shortened lifespan and increased cancer mortality, but at low-dose radiation the ratios of cancer deaths in A-bomb survivors are smaller than those of Japanese averages.[23]
In Taiwan recycled radiocontaminated steel was inadvertently used in the construction of over 100 apartment buildings causing the long-term (10 years) exposure of 10,000 people. The average dose rate was 50 mSv/year and a subset of the population (1,000 people) received a total dose of over 4,000 mSv over ten years. In the widely used Linear No Threshold (LNT) theory used by regulatory bodies, the expected cancer deaths in this population would have been 302 with 70 caused by the extra ionizing radiation with the remainder caused by natural background radiation. However the observed cancer rate was quite low at 7 cancer deaths when 232 would be predicted by the LNT theory had they not been exposed to the radiation from the building materials. Ionizing radiation hormesis appears to be at work. Described by Professor Charles L. Sanders, Korea Advanced Institute of Science and Technology.[24]
Chemical and ionizing radiation combined
No experiment can be performed in perfect isolation. Thick lead shielding around a chemical dose experiment to rule out the effects of ionizing radiation is built and rigorously controlled for in the laboratory, and certainly not the field. Likewise the same applies for ionizing radiation studies. Ionizing radiation is released when an unstable particle releases radiation, creating two new substances and energy in the form of an electromagnetic wave. The resulting materials are then free to interact with any environmental elements, and the energy released can also be used as a catalyst in further ionizing radiation interactions.[25]
The resulting confusion in the low dose exposure field (radiation and chemical) arise from lack of consideration of this concept as described by Mothersill and Seymory.[26] Mothersill and Seymory state "Most of the arguments about whether radiation is 'good for you' or 'bad for you' fail due to lack of consideration of the hierarchical level at which the effect occurs and because most of the arguments are anthropocentric. For example cell death is seen as a 'bad' effect but if it removes a potentially carcinogenic cell from the population of cells in a tissue it could prevent cancer starting and could be seen as 'good'."
Controversy
Whether hormesis is common or important is controversial as it's counter-intuitive and has been used as a pretext by lobbyists to weaken environmental regulations of some well-known toxic substances in the USA.[27]
At least one peer-reviewed article accepts the idea, claiming that over 600 substances show a U-shaped dose–response relationship. Calabrese and Baldwin wrote: "One percent (195 out of 20,285) of the published articles contained 668 dose-response relationships that met the entry criteria."[28] The idea that low dose effects may be (sometimes strikingly) different is accepted, but that the low dose effect is positive is questionable.
The hypothesis of hormesis has generated the most controversy when applied to ionizing radiation. This hypothesis is called radiation hormesis. For policy making purposes, the commonly accepted model of dose response in radiobiology is the linear no-threshold model (LNT), which assumes a strictly linear dependence between the risk of radiation-induced adverse health effects and radiation dose, implying that there is no safe dose of radiation for humans.
Nonetheless, many countries including Czechia, Germany, Austria, Poland and United States have radon therapy centers whose whole primary operating principle is the assumption of radiation hormesis, or beneficial impact of small doses of radiation on human health. Countries like Germany and Austria at the same time have imposed very strict anti-nuclear regulations, which has been described as radiophobic inconsistency.
The United States National Research Council (part of the National Academy of Sciences),[29] the National Council on Radiation Protection and Measurements (a body commissioned by the United States Congress)[30] and the United Nations Scientific Committee on the Effects of Ionizing Radiation (UNSCEAR) all agree that radiation hormesis is not clearly shown, nor clearly the rule for radiation doses.
A United States-based National Council on Radiation Protection and Measurements stated in 2001 that there is insufficient evidence for radiation hormesis and that radiation protection authorities should continue to apply the LNT model for purposes of risk estimation.[30]
A 2005 report commissioned by the French National Academy concluded that there is sufficient evidence for hormesis occurring at low doses and that LNT should be reconsidered as the methodology used to estimate risks from low level sources of radiation, like deep geological repositories for nuclear waste.[31]
Policy consequences
Regulatory agencies such as the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA), and the Nuclear Regulatory Commission (NRC) traditionally use a linear no-threshold model for carcinogens (including radiation). In the linear model, the assumption is that there is no dosage that has no risk of causing cancer. While this linear approach remains the default , with sufficient mechanistic evidence suggesting a non-linear dose-response, EPA allows for the derivation of a threshold dose (also known as reference dose) below which it is assumed that there is no risk for cancer.
While proponents of hormesis argue that changing to a hormesis model would likely change exposure standards for these toxicants in air, water, food and soil, making the standards less strict, other scientists point out that low dose stimulation can have extremely adverse effects. Similarly, low doses of the phthalate DEHP cause increased allergic responses to allergens, while higher doses have no effect. Wider use of the hormesis model would affect how scientists design and conduct studies and the selection of models that estimate risk. In all likelihood, recognizing that low dose effects can't be predicted from high dose experiments would force a strengthening of public health standards, not their weakening, as hormesis proponents would argue.
Hormesis remains largely unknown to the public. Any policy change ought to consider hormesis first as a public health issue (versus an industrial regulatory issue). This would include the assessment of the public concern regarding exposure to small toxic doses. In addition, impact of hormesis policy change upon the management of industrial risks should be studied.[32]
See also
References
- Mattson, M. P (2007). "Hormesis Defined". Ageing Research Reviews. 7 (1): 1–7. doi:10.1016/j.arr.2007.08.007. PMC 2248601. PMID 18162444.
- Kaiser, Jocelyn (2003). "Sipping from a Poisoned Chalice". Science. 302 (5644): 376–9. doi:10.1126/science.302.5644.376. PMID 14563981.
- Axelrod, Deborah; Burns, Kathy; Davis, Devra; von Larebeke, Nicolas (2004). "'Hormesis'—An Inappropriate Extrapolation from the Specific to the Universal". International Journal of Occupational and Environmental Health. 10 (3): 335–9. doi:10.1179/oeh.2004.10.3.335. hdl:1854/LU-867581. PMID 15473091.
- Calabrese, Edward J. (2004). "Hormesis: A revolution in toxicology, risk assessment and medicine". EMBO Reports. 5 (Suppl 1): S37–40. doi:10.1038/sj.embor.7400222. PMC 1299203. PMID 15459733.
- Bethell, Tom (2005). The Politically Incorrect Guide to Science. USA: Regnery Publishing. pp. 58–61. ISBN 978-0-89526-031-4.
- Radak, Zsolt; Chung, Hae Y.; Koltai, Erika; Taylor, Albert W.; Goto, Sataro (2008). "Exercise, oxidative stress and hormesis". Ageing Research Reviews. 7 (1): 34–42. doi:10.1016/j.arr.2007.04.004. PMID 17869589.
- Ristow, Michael; Zarse, Kim (2010). "How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis)". Experimental Gerontology. 45 (6): 410–8. doi:10.1016/j.exger.2010.03.014. PMID 20350594.
- Calabrese, Edward J.; Cook, Ralph (2006). "The Importance of Hormesis to Public Health". Environmental Health Perspectives. 114 (11): 1631–5. doi:10.1289/ehp.8606. JSTOR 4091789. PMC 1665397. PMID 17107845.
- Fillmore, Kaye Middleton; Kerr, William C.; Stockwell, Tim; Chikritzhs, Tanya; Bostrom, Alan (2006). "Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies". Addiction Research & Theory. 14 (2): 101–32. doi:10.1080/16066350500497983. Lay summary – University of California, San Francisco (March 30, 2006).
- Fillmore, Kaye Middleton; Stockwell, Tim; Chikritzhs, Tanya; Bostrom, Alan; Kerr, William (2007). "Moderate Alcohol Use and Reduced Mortality Risk: Systematic Error in Prospective Studies and New Hypotheses". Annals of Epidemiology. 17 (5): S16–23. doi:10.1016/j.annepidem.2007.01.005. PMID 17478320.
- Castro, Paola V.; Khare, Shilpi; Young, Brian D.; Clarke, Steven G. (2012). Singh, Shree Ram (ed.). "Caenorhabditis elegans Battling Starvation Stress: Low Levels of Ethanol Prolong Lifespan in L1 Larvae". PLoS ONE. 7 (1): e29984. Bibcode:2012PLoSO...7E9984C. doi:10.1371/journal.pone.0029984. PMC 3261173. PMID 22279556.
- Heinz, Gary H.; Hoffman, David J.; Klimstra, Jon D.; Stebbins, Katherine R. (2010). "Enhanced reproduction in mallards fed a low level of methylmercury: An apparent case of hormesis". Environmental Toxicology and Chemistry. 29 (3): 650–3. doi:10.1002/etc.64. PMID 20821490. Lay summary – Science News (March 5, 2010).
- Le Bourg, Eric; Rattan, Suresh, eds. (2008). Mild Stress and Healthy Aging: Applying hormesis in aging research and interventions. ISBN 978-1-4020-6868-3.
- Rattan, S. I. (2008). "Principles and practice of hormetic treatment of aging and age-related diseases". Human & Experimental Toxicology. 27 (2): 151–4. doi:10.1177/0960327107083409. PMID 18480141.
- Rattan, Suresh I.S. (2008). "Hormesis in aging". Ageing Research Reviews. 7 (1): 63–78. doi:10.1016/j.arr.2007.03.002. PMID 17964227.
- Gems, David; Partridge, Linda (2008). "Stress-Response Hormesis and Aging: "That which Does Not Kill Us Makes Us Stronger"". Cell Metabolism. 7 (3): 200–3. doi:10.1016/j.cmet.2008.01.001. PMID 18316025.
- Le Bourg; Rattan, eds. (2008). Mild Stress and Healthy Aging: Applying hormesis in aging research and interventions. ISBN 978-1-4020-6868-3.
- Ali, R. E.; Rattan, SI (2006). "Curcumin's Biphasic Hormetic Response on Proteasome Activity and Heat-Shock Protein Synthesis in Human Keratinocytes". Annals of the New York Academy of Sciences. 1067 (1): 394–9. Bibcode:2006NYASA1067..394A. doi:10.1196/annals.1354.056. PMID 16804017.
- Kyriazis, Marios (2005). "Clinical Anti-Aging Hormetic Strategies". Rejuvenation Research. 8 (2): 96–100. doi:10.1089/rej.2005.8.96. PMID 15929717.
- Kyriazis, Marios (2003). "Practical applications of chaos theory to the modulation of human ageing: Nature prefers chaos to regularity". Biogerontology. 4 (2): 75–90. doi:10.1023/A:1023306419861. PMID 12766532.
- Ristow, M; Zarse, K (2010). "How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis)". Experimental Gerontology. 45 (6): 410–8. doi:10.1016/j.exger.2010.03.014. PMID 20350594.
- Bjelakovic, Goran; Nikolova, D; Gluud, LL; Simonetti, RG; Gluud, C (2007). "Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention: Systematic Review and Meta-analysis". JAMA. 297 (8): 842–57. doi:10.1001/jama.297.8.842. PMID 17327526.
- Sutou, S. (2018). Low-dose radiation from A-bombs elongated lifespan and reduced cancer mortality relative to un-irradiated individuals. Genes and Environment, 40(1), 26. https://doi.org/10.1186/s41021-018-0114-3
- Sanders, Charles (2010). Radiation Hormesis and the Linear-No-Threshold Assumption. Radiation Hormesis and the Linear-No-Threshold Assumption by Charles L Sanders. p. 47. Bibcode:2010rhln.book.....S. ISBN 978-3-642-03719-1.
- "Ionizing radiation, health effects and protective measures". World Health Organization. Retrieved 2017-02-16.
- Mothersill C, Seymour C (2009). "Implications for environmental health of multiple stressors". Journal of Radiological Protection. 29 (2A): A21–8. Bibcode:2009JRP....29...21M. doi:10.1088/0952-4746/29/2A/S02. PMID 19454807.
- Facebook; Twitter; options, Show more sharing; Facebook; Twitter; LinkedIn; Email; URLCopied!, Copy Link; Print (2019-02-19). "Scientist says some pollution is good for you — a disputed claim Trump's EPA has embraced". Los Angeles Times. Retrieved 2020-08-11.
- Calabrese EJ, Baldwin LA (2001). "The frequency of U-shaped dose responses in the toxicological literature". Toxicological Sciences. 62 (2): 330–8. doi:10.1093/toxsci/62.2.330. PMID 11452146.
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council (2005). Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. National Academies Press. ISBN 978-0-309-09156-5.
- Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing Radiation. National Council on Radiation Protection and Measurements. 2001. ISBN 978-0-929600-69-7.
- Tubiana, Maurice (2005). "Dose–effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: The joint report of the Académie des Sciences (Paris) and of the Académie Nationale de Médecine". International Journal of Radiation Oncology • Biology • Physics. 63 (2): 317–9. doi:10.1016/j.ijrobp.2005.06.013. PMID 16168825.
- Poumadere, M. (2003). Hormesis: public health policy, organizational safety and risk communication. Human & experimental toxicology, 22(1), 39-41
Further reading
- Mattson, Mark P.; Calabrese, Edward J., eds. (2009). Hormesis: A Revolution in Biology, Toxicology and Medicine. New York: Humana Press. ISBN 978-1-60761-495-1.