Holger Braunschweig
Holger Braunschweig, ML FRSC, is Head and Chair of Inorganic Chemistry at the Julius-Maximilians-University of Würzburg in Würzburg, Germany. He is best known for founding the field of transition metal-boron multiple bonding (transition metal borylenes),[1][2][3][4] the synthesis of the first stable compounds containing boron-boron[5] and boron-oxygen[6] triple bonds, the isolation of the first non-carbon/nitrogen main-group dicarbonyl,[7] and the first fixation of dinitrogen at an element of the p-block of the periodic table.[8] By modifying a strategy pioneered by Prof. Gregory Robinson of the University of Georgia, Braunschweig also discovered the first rational and high-yield synthesis of neutral compounds containing boron-boron double bonds (diborenes).[5][9][10] In 2016 Braunschweig isolated the first compounds of beryllium in the oxidation state of zero.[11]
Holger Braunschweig | |
|---|---|
 | |
| Born | 1961 |
| Nationality | German |
| Alma mater | RWTH Aachen University |
| Known for | Organoboron chemistry, Borylene Chemistry, Diborynes, Diborenes |
| Awards | 2009 DFG Gottfried Wilhelm Leibniz Prize, 2014 RSC Main Group Award, 2016 GDCh Alfred Stock Memorial Prize. |
| Scientific career | |
| Fields | Chemistry, Inorganic Chemistry, Organometallic Chemistry, Main-Group Chemistry, Organoboron chemistry |
| Institutions | University of Würzburg |
| Website | https://www.braunschweiggroup.de/ |
Education and research career
Braunschweig obtained his Ph.D. and Habilitation from RWTH Aachen with P. Paetzold and worked as a postdoctoral researcher with Michael F. Lappert, FRS, at the University of Sussex, Brighton. After two years at Imperial College London as Senior Lecturer and Reader he took up a Chair of Inorganic Chemistry at the Julius-Maximilians-University of Würzburg in 2002, and is now also the founding director of the Institute for Sustainable Chemistry & Catalysis with Boron (ICB).
Professional achievements
In 2009 Braunschweig was awarded the Gottfried Wilhelm Leibniz Prize of the German Research Foundation (DFG) – the highest German-based research prize. He was also awarded the 2014 RSC Main Group Award and the 2016 Alfred Stock Memorial Prize of the German Society of Chemists. He is a Fellow of the Royal Society of Chemistry, a member of the German National Academy of Science (Leopoldina), the Bavarian Academy of Sciences and Humanities, and the North Rhine-Westphalian Academy of Sciences, Humanities and the Arts.
References
- Braunschweig, Holger; Wagner, Trixie (1995-04-13). "Synthesis and Structure of the First Transition Metal Borylene Complexes". Angewandte Chemie International Edition in English. 34 (7): 825–826. doi:10.1002/anie.199508251. ISSN 1521-3773.
- Braunschweig, Holger; Kollann, Carsten; Englert, Ulli (1998-12-04). "Synthesis and Structure of the First Terminal Borylene Complexes". Angewandte Chemie International Edition. 37 (22): 3179–3180. doi:10.1002/(sici)1521-3773(19981204)37:22<3179::aid-anie3179>3.0.co;2-z. ISSN 1521-3773.
- Braunschweig, Holger; Dewhurst, Rian D.; Schneider, Achim (2010-07-14). "Electron-Precise Coordination Modes of Boron-Centered Ligands". Chemical Reviews. 110 (7): 3924–3957. doi:10.1021/cr900333n. ISSN 0009-2665. PMID 20235583.
- Braunschweig, Holger; Dewhurst, Rian D.; Gessner, Viktoria H. (2013-03-25). "Transition metal borylene complexes". Chemical Society Reviews. 42 (8): 3197–208. doi:10.1039/c3cs35510a. ISSN 1460-4744. PMID 23403460.
- Braunschweig, Holger; Dewhurst, Rian D.; Hammond, Kai; Mies, Jan; Radacki, Krzysztof; Vargas, Alfredo (2012-06-15). "Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond". Science. 336 (6087): 1420–1422. Bibcode:2012Sci...336.1420B. doi:10.1126/science.1221138. ISSN 0036-8075. PMID 22700924.
- Braunschweig, Holger; Radacki, Krzysztof; Schneider, Achim (2010-04-16). "Oxoboryl Complexes: Boron−Oxygen Triple Bonds Stabilized in the Coordination Sphere of Platinum". Science. 328 (5976): 345–347. Bibcode:2010Sci...328..345B. doi:10.1126/science.1186028. ISSN 0036-8075. PMID 20395506.
- Braunschweig, Holger; Dewhurst, Rian D.; Hupp, Florian; Nutz, Marco; Radacki, Krzysztof; Tate, Christopher W.; Vargas, Alfredo; Ye, Qing (2015). "Multiple complexation of CO and related ligands to a main-group element". Nature. 522 (7556): 327–330. Bibcode:2015Natur.522..327B. doi:10.1038/nature14489. PMID 26085273.
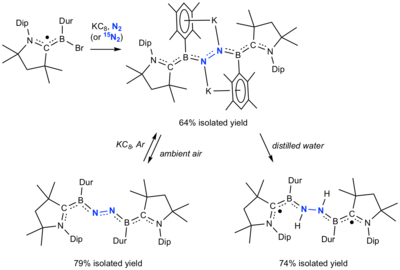
- Légaré, Marc-André; Bélanger-Chabot, Guillaume; Dewhurst, Rian D.; Welz, Eileen; Krummenacher, Ivo; Engels, Bernd; Braunschweig, Holger (2018-02-23). "Nitrogen fixation and reduction at boron". Science. 359 (6378): 896–900. Bibcode:2018Sci...359..896L. doi:10.1126/science.aaq1684. ISSN 0036-8075. PMID 29472479.
- Braunschweig, Holger; Dewhurst, Rian D. (2013-03-25). "Single, Double, Triple Bonds and Chains: The Formation of Electron-Precise B-B Bonds". Angewandte Chemie International Edition. 52 (13): 3574–3583. doi:10.1002/anie.201208189. ISSN 1521-3773. PMID 23362015.
- Arrowsmith, Merle; Braunschweig, Holger; Stennett, Tom E. (2017-01-02). "Formation and Reactivity of Electron-Precise B−B Single and Multiple Bonds". Angewandte Chemie International Edition. 56 (1): 96–115. doi:10.1002/anie.201610072. ISSN 1521-3773. PMID 27860056.
- Arrowsmith, Merle; Braunschweig, Holger; Celik, Mehmet Ali; Dellermann, Theresa; Dewhurst, Rian D.; Ewing, William C.; Hammond, Kai; Kramer, Thomas; Krummenacher, Ivo (2016). "Neutral zero-valent s-block complexes with strong multiple bonding". Nature Chemistry. 8 (9): 890–894. Bibcode:2016NatCh...8..890A. doi:10.1038/nchem.2542. PMID 27334631.