Hemideina thoracica
Hemideina thoracica, commonly known as the Auckland tree wētā or tokoriro[1][2] is a cricket-like insect (within the family Anostostomatidae).[2][3] It is endemic to New Zealand and is found over most of the North Island, except for the Wellington region and regions 900 metres above sea level.[4][5][6] This species is an arboreal, herbivorous[7][8][9] generalist however, it is also thought to be polyphagous[10] and is found in all wooded habitats, including forest, scrub and suburban gardens.[6][11][12]


| Hemideina thoracica | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Orthoptera |
| Suborder: | Ensifera |
| Family: | Anostostomatidae |
| Genus: | Hemideina |
| Species: | H. thoracica |
| Binomial name | |
| Hemideina thoracica (White, 1846) | |
| Synonyms | |
| |
H. thoracica is morphologically uniform but chromosomally polymorphic.[13][14] It comprises at least eight chromosomal races with diploid numbers from 2n=11 (XO) to 2n=23 (XO). There are hybrid zones where some of the chromosomal races meet[14]. Phylogenetically, it is most closely related to the other North Island species (H. crassidens and H. trewicki).[15] The conservation status of H. thoracica is "not threatened"[16][17] however, the chromosome race on Karikari Peninsular (2n=23/24) is listed as "nationally vulnerable".[17]
Taxonomy

Hemideina thoracica was first described by Scottish zoologist Adam White in 1846, but at that time included in the genus Deinacrida.[18] It was later made the type species for the wētā genus Hemideina, described in 1869 by Walker.[18]
Habitat and distribution
The Auckland tree wētā, Hemideina thoracica is endemic to New Zealand and has a wide distribution over the northern two-thirds of the North Island.[5] It is abundant in central and north North Island where it inhabits forest or scrub at lowland elevation.[19] As a nocturnal and arboreal herbivore[19] this species uses tree cavities to rest and conceal itself in during the day before emerging at night to feed[8][2][5][6]. It is parapatric with two other Hemideina spp. in the North Island: H. crassidens and H. trewicki [5] and in warmer areas it is thought to competitively exclude H. crassidens which is distributed in the lower North Island and north-west of the South Island.[5][6]
Diet
Like other tree wētā Hemideina thoracica forages arboreally[9] at night, eating mostly leaves and some fruit and seeds from a range of different plants[12][7]. Recently, it has been suggested that this species feeds selectively on a range of plant species and is omnivorous with invertebrates and fruit and seeds included in the diet.[10]. Plants are selectively eaten with species such as Mahoe (Melicytus ramiflorus) or Karamu (Coprosma robusta) preferred.[2][7][9] However, Auckland tree wētā also feed on small insects[9][2] and are thought to be polyphagous[10]. H. thoracica inhabiting higher elevation sites have been shown to consume more invertebrates and fewer plant species than those at low elevation habitats.[10]
Morphology
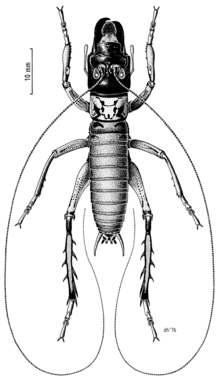
Hemideina thoracica are large-bodied as adults (3–7 g), being up to 40mm in length.[2][7] The abdomen is brown and the pronotum pale with dark hieroglyph-like markings.[6] This body colouration makes H. thoracica easily distinguishable from the Wellington tree wētā (H. crassidens) with which it is parapatric.[6] As in other Hemideina spp. spines are present on the hind legs which function in defense.[2][11] Morphometric analysis of spination patterns has shown that both H. thoracica and H. crassidens lack the mid-tibial ‘1/3 back’ spine which differentiates these species from all other Hemideina spp..[11] Hemideina thoracica has the thinnest femur and tibia compared with all other New Zealand tree wētā (Hemideina spp).[11] Adults are sexually dimorphic with males having enlarged mandibles used for fighting other males.[20][21]
Cytogenetics

Hemideina thoracica is chromosomally polymorphic meaning that differential chromosomal arrangement occurs among populations of this species.[14][13] Nine distinct chromosome races each comprising a different karyotype have been described and these range in diploid numbers from 2n=11 (XO) to 2n=23 (XO).[14][13] Five different hybrid zones have been located where, in different combinations six of the nine chromosome races come into contact with one another. [14] Three of these zones (Mt. Camel, Karikari and Waitangi) involve northern chromosome races which likely originated in the Pliocene, whereas the southern races in the Bream Bay and Taupo zones are likely to be much younger. [14][22] Despite having differing chromosome numbers, morphology of H. thoracica is uniform[13][14] (see 'Morphology' above).
Behaviour

Hemideina thoracica is capable of producing sound using stridulation of the metathoracic legs and the abdomen.[23] Both males and females produce a rasping sound when disturbed by raising and extending the legs above the body followed by a defensive kick while tightly holding the legs against the abdomen.[23] Using abdominal oscillation, males also generate an intraspecific song when in close contact with one another.[23] However, H. thoracica has poor mate recognistion systems and forms sterile hybrids with H. crassidens and H. trewicki where they are sympatric in the southern North Island.[24]
As a nocturnal species, H. thoracica occupy cavities during the day[2][5][6][8] and occupancy patterns have been shown to be influenced by both season and sex of previous occupants.[20] Females tend to avoid cavities in which other females reside and are found in cavities alone however, this changes during summer when females form harems[20] Males and females which were previously living apart begin living together in early summer.[20]
Breeding
It has been suggested that Auckland tree wētā have a polygynandrous mating system whereby both males and females mate with multiple partners.[20] This species is a hemimetabolous insect whose eggs hatch in Spring with a minimum of eight instars required to reach adulthood.[20] Females lay eggs in the soil and provide no maternal care.
Conservation

The conservation status of Hemideina thoracica is "not threatened"[16][17] however, the chromosome race on Karikari Peninsular (2n=23/24) is listed as "nationally vulnerable".[17] Artificial refuges have been used to monitor populations of H. thoracica and H. crassidens[25] and could potentially be used in conservation management of these species by providing available habitat.[8][25]
References
- "Wētā". Science Learning Hub. Retrieved 4 July 2019.
- "Weta (Tree) Auckland (Hemideina thoracica)". www.terrain.net.nz. T.E.R:R.A.I.N - Taranaki Educational Resource: Research, Analysis and Information Network. Retrieved 18 August 2017.
- Johns, P. M. (1997). "The Gondwanaland Weta: Family Anostostomatidae (Formerly in Stenopelmatidae, Henicidae or Mimnermidae): Nomenclatural Problems, World Checklist, New Genera and Species". Journal of Orthoptera Research (6): 125–138. doi:10.2307/3503546. ISSN 1082-6467. JSTOR 3503546.
- Pratt, Renae C; Morgan-Richards, Mary; Trewick, Steve A (2008). "Diversification of New Zealand weta (Orthoptera: Ensifera: Anostostomatidae) and their relationships in Australasia". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1508): 3427–3437. doi:10.1098/rstb.2008.0112. ISSN 0962-8436. PMC 2607373. PMID 18782727.
- Bulgarella, Mariana; Trewick, Steven A.; Minards, Niki A.; Jacobson, Melissa J.; Morgan‐Richards, Mary (2014). "Shifting ranges of two tree weta species (Hemideina spp.): competitive exclusion and changing climate". Journal of Biogeography. 41 (3): 524–535. doi:10.1111/jbi.12224. ISSN 1365-2699.
- Trewick, Steven A.; Morgan‐Richards, Mary (1995). "On the distribution of tree weta in the North Island, New Zealand". Journal of the Royal Society of New Zealand. 25 (4): 485–493. doi:10.1080/03014223.1995.9517498. ISSN 0303-6758.
- Wehi, Priscilla M.; Hicks, Brendan J. (2010). "Isotopic fractionation in a large herbivorous insect, the Auckland tree weta". Journal of Insect Physiology. 56 (12): 1877–1882. doi:10.1016/j.jinsphys.2010.08.005. ISSN 0022-1910. PMID 20709068.
- Wehi, Priscilla M.; Jorgensen, Murray; Morgan, Dai K.J. (2015). "Predictors of relative abundance of tree weta (Hemideina thoracica) in an urban forest remnant". New Zealand Journal of Ecology. 39 (2): 280–285. ISSN 0110-6465. JSTOR 26198721.
- "Tree weta ecology". wetageta.massey.ac.nz. Retrieved 30 June 2019.
- Brown, Matthew B. G. J.; Gemmill, Chrissen E. C.; Miller, Steven; Wehi, Priscilla M. (2018). "Diet selectivity in a terrestrial forest invertebrate, the Auckland tree wētā, across three habitat zones". Ecology and Evolution. 8 (5): 2495–2503. doi:10.1002/ece3.3763. ISSN 2045-7758. PMC 5838035. PMID 29531670.
- Field, L. H. (2001). The Biology of Wetas, King Crickets and Their Allies. CABI. ISBN 9780851997827.
- "Tree weta". wetageta.massey.ac.nz. Retrieved 3 September 2017.
- Morgan-Richards, M (1997). "Intraspecific karyotype variation is not concordant with allozyme variation in the Auckland tree weta of New Zealand, Hemideina thoracica (Orthoptera: Stenopelmatidae)". Biological Journal of the Linnean Society. 60 (4): 423–442. doi:10.1111/j.1095-8312.1997.tb01505.x.
- Morgan-Richards, Mary; Wallis, Graham P. (2003). "A Comparison of Five Hybrid Zones of the Weta Hemideina thoracica (Orthoptera: Anostostomatidae): Degree of Cytogenetic Differentiation Fails to Predict Zone Width". Evolution. 57 (4): 849–861. doi:10.1111/j.0014-3820.2003.tb00296.x. JSTOR 3094621. PMID 12778554.
- Twort, Victoria G; Newcomb, Richard D; Buckley, Thomas R (1 April 2019). Bryant, David (ed.). "New Zealand Tree and Giant Wētā (Orthoptera) Transcriptomics Reveal Divergent Selection Patterns in Metabolic Loci". Genome Biology and Evolution. 11 (4): 1293–1306. doi:10.1093/gbe/evz070. ISSN 1759-6653. PMC 6486805. PMID 30957857.
- Trewick, S.A., Johns, P., Hitchmough, R., Rolfe, J. & Stringer, I. Conservation status of New Zealand orthoptera, 2014. New Zealand Threat Classification Series 16. ISBN 9780478150872. OCLC 990218289.CS1 maint: multiple names: authors list (link)
- "The conservation status of New Zealand Orthoptera". ResearchGate. Retrieved 3 September 2017.
- Hemideina at OSF
- Morgan-Richards, Mary; Trewick, Steven A. (2004). "Phylogenetics of New Zealand's tree, giant and tusked weta (Orthoptera: Anostostomatidae): evidence from mitochondrial DNA". Journal of Orthoptera Research. 13 (2): 185–196. doi:10.1665/1082-6467(2004)013[0185:PONZTG]2.0.CO;2. ISSN 1082-6467.
- Morgan-Richards, Mary; Jorgensen, Murray A.; Wehi, Priscilla M. (2013). "Sex- and season-dependent behaviour in a flightless insect, the Auckland tree weta (Hemideina thoracica)". 37 (1): 75–83. ISSN 0110-6465. Cite journal requires
|journal=(help) - Wehi, P. M.; Nakagawa, S.; Trewick, S. A.; Morgan‐Richards, M. (2011). "Does predation result in adult sex ratio skew in a sexually dimorphic insect genus?". Journal of Evolutionary Biology. 24 (11): 2321–2328. doi:10.1111/j.1420-9101.2011.02366.x. ISSN 1420-9101. PMID 21848984.
- Wallis, Graham P.; Trewick, Steven A.; Morgan-Richards, Mary (2001). "Chromosome races with Pliocene origins: evidence from mtDNA". Heredity. 86 (3): 303–312. doi:10.1046/j.1365-2540.2001.00828.x. ISSN 1365-2540. PMID 11488967.
- McVean, Alistair (1986). "The song of the New Zealand weta, Hemideina thoracica (Orthoptera: Stenopelmatidae)". Journal of Zoology. 208 (2): 171–190. doi:10.1111/j.1469-7998.1986.tb01506.x.
- Mckean, Natasha E.; Trewick, Steven A.; Morgan-Richards, Mary (2016). "Little or no gene flow despite F 1 hybrids at two interspecific contact zones". Ecology and Evolution. 6 (8): 2390–2404. doi:10.1002/ece3.1942. PMC 4783458. PMID 27066230.
- Bleakley C, Stringer I, Robertson A, Hedderley D (2006). Design and use of artificial refuges for monitoring adult tree weta, Hemideina crassidens and H. thoracica. Science & Technical Pub., Dept. of Conservation. ISBN 0478140622. OCLC 66436455.CS1 maint: multiple names: authors list (link)