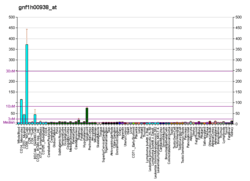
HAVCR2
Hepatitis A virus cellular receptor 2 (HAVCR2), also known as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), is a protein that in humans is encoded by the HAVCR2 gene. HAVCR2 was first described in 2002 as a cell surface molecule expressed on IFNγ producing CD4+ Th1 and CD8+ Tc1 cells.[5][6] Later, the expression was detected in Th17 cells,[7] regulatory T-cells,[8] and innate immune cells (dendritic cells, NK cells, monocytes).[9]
Structure
HAVCR2 belongs to TIM family cell surface receptor proteins. These proteins share a similar structure, in which the extracellular region consists of membrane distal single variable immunoglobulin domain (IgV) and a glycosylated mucin domain of variable length located closer to the membrane.[10] Intracellular domain of HAVCR2 is called C-terminal cytoplasmic tail. It contains five conserved tyrosine residues that interact with multiple components of T-cell receptor (TCR) complex[11][12] and negatively regulates its function.[13]
Function
HAVCR2 is an immune checkpoint and together with other inhibitory receptors including programmed cell death protein 1 (PD-1) and lymphocyte activation gene 3 protein (LAG3) mediate the CD8+ T-cell exhaustion.[14] HAVCR2 has also been shown as a CD4+ Th1-specific cell surface protein that regulates macrophage activation and enhances the severity of experimental autoimmune encephalomyelitis in mice.[5]
HAVCR2 is primarily activated by galectin-9.[15] The engagement leads to stimulation of an influx of calcium to intracellular space and induction of programmed cell death, apoptosis.[16] As a consequence, a suppression of Th1 and Th17 responses and induction of immune tolerance occurs. In addition to galectin-9, a couple other ligands have been identified, such as phospatidyl serine (PtdSer),[17] High Mobility Group Protein 1 (HMGB1)[18] and Carcinoembryonic Antigen Related Cell Adhesion Molecule 1 (CEACAM1).[19] The binding of PtdSer has been shown to cause an uptake of apoptotic cells and reduced cross presentation of dying cell-associated antigens by dendritic cells.[20] The binding of HMGB1 can interfere with nucleic acid stimulation and suppresses activation of innate immune response.[18] The role of CEACAM1 engagement is still not clear.
Clinical significance
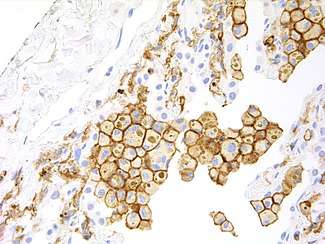
HAVCR2 expression is up regulated in tumor-infiltrating lymphocytes in lung,[8] gastric,[21] head and neck cancer,[22] schwannoma,[23] melanoma[24] and follicular B-cell non-Hodgkin lymphoma.[25]
The HAVCR2 pathway may interact with the PD-1 pathway in the dysfunctional CD8+ T cells and Tregs in cancer.[26][8] HAVCR2 is mainly expressed on activated CD8+ T cells and suppresses macrophage activation following PD-1 inhibition.[27] Upregulation was observed in tumors progressing after anti-PD-1 therapy.[28] This seems to be a form of adaptive resistance to immunotherapy. Multiple phase 1/2 clinical trials with anti-HAVCR2 monoclonal antibodies (LY3321367,[29] Eli Lilly and Company; MBG453,[30] Novartis Pharmaceuticals; TSR-022,[31] Tesaro, Inc.) in combination with anti-PD-1 or anti-PD-L1 therapies are ongoing.
The role of HAVCR2 in the T-cell dysfunction has been investigated in chronic viral infections.[32]
References
- GRCh38: Ensembl release 89: ENSG00000135077 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020399 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK (January 2002). "Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease". Nature. 415 (6871): 536–41. doi:10.1038/415536a. PMID 11823861.
- "Entrez Gene: HAVCR2 hepatitis A virus cellular receptor 2".
- Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK (September 2009). "TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines". European Journal of Immunology. 39 (9): 2492–501. doi:10.1002/eji.200939274. PMC 2759376. PMID 19676072.
- Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B (2012). "TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression". PLOS One. 7 (2): e30676. doi:10.1371/journal.pone.0030676. PMC 3281852. PMID 22363469.
- Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, et al. (March 2012). "Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9". Blood. 119 (13): 3064–72. doi:10.1182/blood-2011-06-360321. PMC 3321868. PMID 22323453.
- Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H, Xiao H, Dilorenzo TP, Allison JP, Nathenson SG, Almo SC (March 2007). "T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface". Immunity. 26 (3): 311–21. doi:10.1016/j.immuni.2007.01.016. PMID 17363302.
- Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP (October 2011). "Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways". Molecular and Cellular Biology. 31 (19): 3963–74. doi:10.1128/MCB.05297-11. PMC 3187355. PMID 21807895.
- van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW (December 2006). "A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9". Biochemical and Biophysical Research Communications. 351 (2): 571–6. doi:10.1016/j.bbrc.2006.10.079. PMID 17069754.
- Tomkowicz B, Walsh E, Cotty A, Verona R, Sabins N, Kaplan F, Santulli-Marotto S, Chin CN, Mooney J, Lingham RB, Naso M, McCabe T (2015). "TIM-3 Suppresses Anti-CD3/CD28-Induced TCR Activation and IL-2 Expression through the NFAT Signaling Pathway". PLOS One. 10 (10): e0140694. doi:10.1371/journal.pone.0140694. PMC 4619610. PMID 26492563.
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ (January 2009). "Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection". Nature Immunology. 10 (1): 29–37. doi:10.1038/ni.1679. PMC 2605166. PMID 19043418.
- Wada J, Kanwar YS (February 1997). "Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin". The Journal of Biological Chemistry. 272 (9): 6078–86. doi:10.1074/jbc.272.9.6078. PMID 9038233.
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK (December 2005). "The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity". Nature Immunology. 6 (12): 1245–52. doi:10.1038/ni1271. PMID 16286920.
- DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM (February 2010). "T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells". Journal of Immunology. 184 (4): 1918–30. doi:10.4049/jimmunol.0903059. PMC 3128800. PMID 20083673.
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M (September 2012). "Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1". Nature Immunology. 13 (9): 832–42. doi:10.1038/ni.2376. PMC 3622453. PMID 22842346.
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS (January 2015). "CEACAM1 regulates TIM-3-mediated tolerance and exhaustion". Nature. 517 (7534): 386–90. doi:10.1038/nature13848. PMC 4297519. PMID 25363763.
- Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K (April 2009). "Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation". Blood. 113 (16): 3821–30. doi:10.1182/blood-2008-10-185884. PMID 19224762.
- Lu X, Yang L, Yao D, Wu X, Li J, Liu X, Deng L, Huang C, Wang Y, Li D, Liu J (March 2017). "Tumor antigen-specific CD8+ T cells are negatively regulated by PD-1 and Tim-3 in human gastric cancer". Cellular Immunology. 313: 43–51. doi:10.1016/j.cellimm.2017.01.001. PMID 28110884.
- Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL (2017). "Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer". Oncoimmunology. 6 (1): e1261779. doi:10.1080/2162402X.2016.1261779. PMC 5283618. PMID 28197389.
- Li Z, Liu X, Guo R, Wang P (May 2017). "TIM-3 plays a more important role than PD-1 in the functional impairments of cytotoxic T cells of malignant Schwannomas". Tumour Biology. 39 (5): 1010428317698352. doi:10.1177/1010428317698352. PMID 28475007.
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM (September 2010). "Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients". The Journal of Experimental Medicine. 207 (10): 2175–86. doi:10.1084/jem.20100637. PMC 2947081. PMID 20819923.
- Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM (April 2012). "IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma". The Journal of Clinical Investigation. 122 (4): 1271–82. doi:10.1172/JCI59806. PMC 3314462. PMID 22426209.
- Anderson AC (May 2014). "Tim-3: an emerging target in the cancer immunotherapy landscape". Cancer Immunology Research. 2 (5): 393–8. doi:10.1158/2326-6066.CIR-14-0039. PMID 24795351.
- Dempke WC, Fenchel K, Uciechowski P, Dale SP (2017). "Second- and third-generation drugs for immuno-oncology treatment-The more the better?". European Journal of Cancer. 74: 55–72. doi:10.1016/j.ejca.2017.01.001. PMID 28335888.
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. (February 2016). "Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints". Nature Communications. 7: 10501. doi:10.1038/ncomms10501. PMC 4757784. PMID 26883990.
- Clinical trial number NCT03099109 for "A Study of LY3321367 Alone or With LY3300054 in Participants With Advanced Relapsed/Refractory Solid Tumors" at ClinicalTrials.gov
- Clinical trial number NCT02608268 for "Safety and Efficacy of MBG453 as Single Agent and in Combination With PDR001 in Patients With Advanced Malignancies" at ClinicalTrials.gov
- Clinical trial number NCT02817633 for "Study of TSR-022, an Anti-TIM-3 Monoclonal Antibody, in Patients With Advanced Solid Tumors" at ClinicalTrials.gov
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R (August 2010). "Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection". Proceedings of the National Academy of Sciences of the United States of America. 107 (33): 14733–8. doi:10.1073/pnas.1009731107. PMC 2930455. PMID 20679213.