Tumor-infiltrating lymphocytes
Tumor-infiltrating lymphocytes are white blood cells that have left the bloodstream and migrated towards a tumor. They include T cells and B cells and are part of the larger category of ‘tumor-infiltrating immune cells’ which consist of both mononuclear and polymorphonuclear immune cells, (i.e., T cells, B cells, natural killer cells, macrophages, neutrophils, dendritic cells, mast cells, eosinophils, basophils, etc.) in variable proportions. Their abundance varies with tumor type and stage and in some cases relates to disease prognosis [1][2][3][4][5]
TILs can often be found in the tumor stroma and within the tumor itself. Their functions can dynamically change throughout tumor progression and in response to anticancer therapy[2][3][4][5]
TILs are implicated in killing tumor cells. The presence of lymphocytes in tumors is often associated with better clinical outcomes (after surgery or immunotherapy).[6][7][8][9]
Detection and characteristics
TILs can be found between the tumor cells, as TILs in the stroma surrounding the tumor cells do not count.[10] TILs are often found floating around the tumor without actual penetration or action on the tumor cells. Histologic definitions for TILs vary.
CD3 has been used to detect lymphocytes in tumor samples.[8] Tumor immune infiltration can also be determined using gene expression methods like Microarray or RNA Sequencing through deconvolution methods such as CIBERSORT.[11][12] Such methods allow for systematic TIL enumeration and characterization of the tumor microenvironment in diverse cancer types and across thousands of tumors[12][5], an approach largely led by Ash Alizadeh, Ajit Johnson among others. Detection of gene expression specific for different kind of immune cell populations can then be used to determine the degree of lymphocyte infiltration as has been shown in breast cancer.[13] An active immune environment within the tumor often indicates a better prognosis as can be determined by the Immunological constant of rejection.[14]
Use in autologous cell therapy
They are key to an experimental autologous cell therapy (Contego) for metastatic melanoma.[15] Autologous TIL therapy for metastatic melanoma has broad T cell recognition of both defined and undefined tumor antigens against all human leukocyte antigen (HLA) restrictions. TILs can not only recognize over-expressed self/melanocyte differentiation antigens, such as Melan-A/MART-1 (melanoma-specific), gp100, tyrosinase, and survivin, but TILs can also recognize other unknown antigens specific to the tumor and individual patient.[16]
Use in adoptive T cell transfer therapy
History
The use of TILs as an adoptive cell transfer therapy to treat cancer was pioneered by Dr. Steven Rosenberg and colleagues at the Surgery Branch of the National Cancer Institute (NCI).[17] Rosenberg and colleagues have conducted clinical trials for more than two decades using TIL adoptive cell therapy for melanoma.[18] TIL adoptive cell therapy is now a routine regimen in centers across the world, including MD Anderson Cancer Center, where the objective response rates originally observed at the NCI have been reproduced.[19][20] Several centers currently have established TIL therapy protocols for the treatment of melanoma, including the MD Anderson Cancer Center in Houston, Texas,[17] Ella Institute in Sheba Hospital, Israel,[19] and Copenhagen University Hospital in Herlev, Denmark.[21][22]
Process
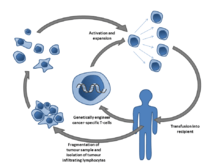
In Adoptive T cell transfer therapy, TILs are expanded ex vivo from surgically resected tumors that have been cut into small fragments or from single cell suspensions isolated from the tumor fragments. Multiple individual cultures are established, grown separately and assayed for specific tumor recognition. TILs are expanded over the course of a few weeks with a high dose of IL-2 in 24-well plates. Selected TIL lines that presented best tumor reactivity are then further expanded in a "rapid expansion protocol" (REP), which uses anti-CD3 activation for a typical period of two weeks. The final post-REP TIL is infused back into the patient. The process can also involve a preliminary chemotherapy regimen to deplete endogenous lymphocytes in order to provide the adoptively transferred TILs with enough access to surround the tumor sites. This chemotherapy regimen is given 7 days before the expanded TIL infusion.[17] This involves pretreatment with a combination of fludarabine and cyclophosphamide. Lympho-depletion is thought to eliminate the negative effects of other lymphocytes that may compete for growth factors and decrease anti-tumor effects of the TILs, depleting regulatory or inhibitory lymphocyte populations.[23]
Clinical Success
The combination of TILs with a high dose of IL-2 presents multiple clinical trials demonstrating rates near 50% or more patients effectively responding.[24] In summary of TIL therapy clinical trials, TIL therapy was found to induce complete and durable regression of metastatic melanoma. Tumor reduction of 50% or more was observed in about half of patients.[25][20][26][19] Some patients experienced complete responses with no detectable tumor remaining years after treatment.[18] In one clinical trials, among the 93 patients treated with TILs, 19 patients had complete remissions that lasted greater than 3 years.[17]
Clinical trials using TILs to treat digestive tract cancers, such as colorectal cancer,[27] and cancers associated with the human papilloma virus (HPV), such as cervical cancer,[28] are ongoing. In colorectal cancer, TILs are associated with microsatellite instability cancers, as may be seen in Lynch syndrome.[29] Also, TILs are associated with most effective immune checkpoint inhibitor therapy in GI cancers.[8][9] They are an important prognostic factor in melanoma and higher levels being associated with a better outcome.[30][31][9] TILs are also associated with better outcomes in epithelial ovarian cancer.[7][9]
The use of TILs to treat other tumor types, including lung, ovarian, bladder, and breast, are under investigation.
Associations with cancer treatments
TIL therapy in combination with prior immunotherapy treatment, such as IL-2 and anti-CTLA4 (ipilimumab) had higher response rates and more durable responses in clinical trials. This suggests a synergistic effect of prior immunotherapy with TIL therapy.[18] Current studies involve investigating the roles of chemotherapy drugs in combination with TIL therapy to assess improved response rates and synergistic efficacy.[32][33]
See also
- Lymphocyte
- Tumor-Infiltrating Dendritic Cell
- Cancer Immunotherapy
- CAR T-Cell therapy
- T-cells
References
- Teixeira, Luis; Rothé, Françoise; Ignatiadis, Michail; Sotiriou, Christos (2016). "Breast Cancer Immunology". Oncology Times. 38 (9): 18–19. doi:10.1097/01.COT.0000483221.52404.e3.
- Hanahan D, Coussens LM (March 2012). "Accessories to the crime: functions of cells recruited to the tumor microenvironment". Cancer Cell. 21 (3): 309–22. doi:10.1016/j.ccr.2012.02.022. PMID 22439926.
- Coussens LM, Zitvogel L, Palucka AK (January 2013). "Neutralizing tumor-promoting chronic inflammation: a magic bullet?". Science. 339 (6117): 286–91. doi:10.1126/science.1232227. PMC 3591506. PMID 23329041.
- Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, Savova V, Zemmour D, Kline J, Siwicki M, Garris C, Pucci F, Liao HW, Lin YJ, Newton A, Yaghi OK, Iwamoto Y, Tricot B, Wojtkiewicz GR, Nahrendorf M, Cortez-Retamozo V, Meylan E, Hynes RO, Demay M, Klein A, Bredella MA, Scadden DT, Weissleder R, Pittet MJ (December 2017). "high neutrophils". Science. 358 (6367): eaal5081. doi:10.1126/science.aal5081. PMC 6343476. PMID 29191879.
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA (August 2015). "The prognostic landscape of genes and infiltrating immune cells across human cancers". Nature Medicine. 21 (8): 938–945. doi:10.1038/nm.3909. PMC 4852857. PMID 26193342.
- Vánky F, Klein E, Willems J, Böök K, Ivert T, Péterffy A, Nilsonne U, Kreicbergs A, Aparisi T (1986). "Lysis of autologous tumor cells by blood lymphocytes tested at the time of surgery. Correlation with the postsurgical clinical course". Cancer Immunology, Immunotherapy. 21 (1): 69–76. doi:10.1007/BF00199380. PMID 3455878.
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (January 2003). "Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer" (PDF). The New England Journal of Medicine. 348 (3): 203–213. doi:10.1056/NEJMoa020177. PMID 12529460.
- Immunotherapy Doubts Fading in GI Cancers. April 2016
- Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- Garg K, Soslow RA (August 2009). "Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma". Journal of Clinical Pathology. 62 (8): 679–84. doi:10.1136/jcp.2009.064949. PMID 19638537.
- Nirmal, Ajit J.; Regan, Tim; Shih, Barbara B.; Hume, David A.; Sims, Andrew H.; Freeman, Tom C. (2018-11-01). "Immune Cell Gene Signatures for Profiling the Microenvironment of Solid Tumors". Cancer Immunology Research. 6 (11): 1388–1400. doi:10.1158/2326-6066.CIR-18-0342. ISSN 2326-6066. PMID 30266715.
- Newman, Aaron M; Liu, Chih Long; Green, Michael R; Gentles, Andrew J; Feng, Weiguo; Xu, Yue; Hoang, Chuong D; Diehn, Maximilian; Alizadeh, Ash A (2015-03-30). "Robust enumeration of cell subsets from tissue expression profiles". Nature Methods. 12 (5): 453–457. doi:10.1038/nmeth.3337. ISSN 1548-7091. PMC 4739640. PMID 25822800.
- Bedognetti D, Hendrickx W, Marincola FM, Miller LD (November 2015). "Prognostic and predictive immune gene signatures in breast cancer". Current Opinion in Oncology. 27 (6): 433–44. doi:10.1097/cco.0000000000000234. PMID 26418235.
- Bedognetti D, Hendrickx W, Ceccarelli M, Miller LD, Seliger B (April 2016). "Disentangling the relationship between tumor genetic programs and immune responsiveness". Current Opinion in Immunology. 39: 150–8. doi:10.1016/j.coi.2016.02.001. PMID 26967649.
- "Genesis Biopharma expands clinical focus to develop Contego for Stage IV metastatic melanoma". June 2011.
- Restifo NP, Dudley ME, Rosenberg SA (March 2012). "Adoptive immunotherapy for cancer: harnessing the T cell response". Nature Reviews. Immunology. 12 (4): 269–81. doi:10.1038/nri3191. PMC 6292222. PMID 22437939.
- Lizée G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P (2013-01-14). "Harnessing the power of the immune system to target cancer". Annual Review of Medicine. 64 (1): 71–90. doi:10.1146/annurev-med-112311-083918. PMID 23092383.
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME (July 2011). "Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy". Clinical Cancer Research. 17 (13): 4550–7. doi:10.1158/1078-0432.CCR-11-0116. PMC 3131487. PMID 21498393.
- Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J (May 2010). "Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients". Clinical Cancer Research. 16 (9): 2646–55. doi:10.1158/1078-0432.CCR-10-0041. PMID 20406835.
- Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson VE, McMannis JD, Shpall E, Prieto V, Papadopoulos N, Kim K, Homsi J, Bedikian A, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P (December 2012). "Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients". Clinical Cancer Research. 18 (24): 6758–70. doi:10.1158/1078-0432.CCR-12-1177. PMC 3525747. PMID 23032743.
- Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met Ö, Hölmich LR, Andersen RS, Hadrup SR, Andersen MH, thor Straten P, Svane IM (August 2012). "Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients". Journal of Translational Medicine. 10: 169. doi:10.1186/1479-5876-10-169. PMC 3514199. PMID 22909342.
- Donia M, Hansen M, Sendrup SL, Iversen TZ, Ellebæk E, Andersen MH, Straten P, Svane IM (February 2013). "Methods to improve adoptive T-cell therapy for melanoma: IFN-γ enhances anticancer responses of cell products for infusion". The Journal of Investigative Dermatology. 133 (2): 545–52. doi:10.1038/jid.2012.336. PMID 23014345.
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP (October 2005). "Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells". The Journal of Experimental Medicine. 202 (7): 907–12. doi:10.1084/jem.20050732. PMC 1397916. PMID 16203864.
- Dudley ME, Rosenberg SA (September 2003). "Adoptive-cell-transfer therapy for the treatment of patients with cancer". Nature Reviews. Cancer. 3 (9): 666–75. doi:10.1038/nrc1167. PMC 2305722. PMID 12951585.
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA (November 2008). "Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens". Journal of Clinical Oncology. 26 (32): 5233–9. doi:10.1200/JCO.2008.16.5449. PMC 2652090. PMID 18809613.
- Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, Kudchadkar R, Zager J, Gibney G, Sondak VK, Weber J, Mulé JJ, Sarnaik AA (October 2012). "Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma". Journal of Immunotherapy. 35 (8): 615–20. doi:10.1097/CJI.0b013e31826e8f5f. PMC 4467830. PMID 22996367.
- Clinical trial number NCT01174121 for "A Phase II Study Using Short-Term Cultured, CD8+-Enriched Autologous Tumor-infiltrating Lymphocytes Following a Lymphocyte Depleting Regimen in Metastatic Digestive Tract Cancers" at ClinicalTrials.gov
- Clinical trial number NCT01585428 for "A Phase II Study of Lymphodepletion Followed by Autologous Tumor-Infiltrating Lymphocytes and High-Dose Adesleukin for Human Papillomavirus-Associated Cancers" at ClinicalTrials.gov
- Iacopetta B, Grieu F, Amanuel B (December 2010). "Microsatellite instability in colorectal cancer" (PDF). Asia-Pacific Journal of Clinical Oncology. 6 (4): 260–9. doi:10.1111/j.1743-7563.2010.01335.x. PMID 21114775.
- Spatz; et al. (2007). "Protective effect of a brisk tumor infiltrating lymphocyte infiltrate in melanoma: An EORTC melanoma group study". Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 8519.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (September 2006). "Type, density, and location of immune cells within human colorectal tumors predict clinical outcome". Science. 313 (5795): 1960–4. doi:10.1126/science.1129139. PMID 17008531.
- Mbofung RM, McKenzie JA, Malu S, Zhang M, Peng W, Liu C, Kuiatse I, Tieu T, Williams L, Devi S, Ashkin E, Xu C, Huang L, Zhang M, Talukder AH, Tripathi SC, Khong H, Satani N, Muller FL, Roszik J, Heffernan T, Allison JP, Lizee G, Hanash SM, Proia D, Amaria R, Davis RE, Hwu P (September 2017). "HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes". Nature Communications. 8 (1): 451. doi:10.1038/s41467-017-00449-z. PMC 5587668. PMID 28878208.
- McKenzie JA, Mbofung RM, Malu S, Zhang M, Ashkin E, Devi S, Williams L, Tieu T, Peng W, Pradeep S, Xu C, Zorro Manrique S, Liu C, Huang L, Chen Y, Forget MA, Haymaker C, Bernatchez C, Satani N, Muller F, Roszik J, Kalra A, Heffernan T, Sood A, Hu J, Amaria R, Davis RE, Hwu P (December 2017). "The Effect of Topoisomerase I Inhibitors on the Efficacy of T-Cell-Based Cancer Immunotherapy". Journal of the National Cancer Institute. 110 (7): 777–786. doi:10.1093/jnci/djx257. PMC 6037061. PMID 29267866.
External links
- Tumor infiltrating lymphocyte entry in the public domain NCI Dictionary of Cancer Terms
- Lion Biotechnologies, Inc.
