Gymnemic acid
Gymnemic acids are a class of chemical compounds isolated from the leaves of Gymnema sylvestre (Asclepiadaceae). They are anti-sweet compounds, or sweetness inhibitors.[1] After chewing the leaves, solutions sweetened with sugar taste like water.
Chemically, gymnemic acids are triterpenoid glycosides. The central structure is the aglycone gymnemagenin (C30H50O6).[2] This is adorned with a sugar such as glucuronic acid and with various ester groups. These variations give rise to the different gymnemic acids.[3] More than 20 homologs of gymnemic acid are known.[4]
Gymnemic acid I has the highest anti-sweet properties. It suppresses the sweetness of most of the sweeteners including intense artificial sweeteners such as aspartame and natural sweeteners such as thaumatin, a sweet protein. The anti-sweet activity is reversible, but sweetness recovery on the tongue can take more than 10 minutes.[5]
Example chemical structures
| Gymnemic acids[5] | |||||
|---|---|---|---|---|---|
| Chemical structure | 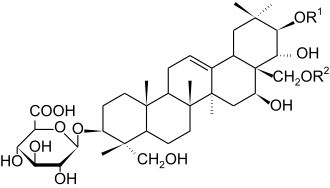 | ||||
| Name | Gymnemic acid I | Gymnemic acid II | Gymnemic acid III | Gymnemic acid IV | |
| R1 | tigloyl | 2-methylbutanoyl | 2-methylbutanoyl | tigloyl | |
| R2 | acetyl | acetyl | H | H | |
| CAS Number | 122168-40-5 | 122144-48-3 | 122074-65-1 | 121903-96-6 | |
| PubChem ID | 11953919 | 91617872 | 14264066 | 14264063 | |
| Chemical formula | C43H66O14 | C43H68O14 | C41H66O13 | C41H64O13 | |
| Molar mass (g/mol) | 806.98 | 809.00 | 766.97 | 764.95 | |
See also
Other anti-sweeteners:
References
- Stoecklin, Walter (1969). "Chemistry and physiological properties of gymnemic acid, the antisaccharine principle of the leaves of Gymnema sylvestre". Journal of Agricultural and Food Chemistry. 17 (4): 704. doi:10.1021/jf60164a011.
- CID 10051937 from PubChem
- Sheng, Huaming; Sun, Hongbin (2011). "Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases". Natural Product Reports. 28 (3): 543. doi:10.1039/C0NP00059K. PMID 21290067.
- AD kinghorn and CM Compadre (1991). L O'Brien Nabors (ed.). Less common high-potency sweeteners. Alternative Sweeteners (2nd ed.). New York: Marcel Dekker. ISBN 0-8247-8475-8.
- Kurihara, Yoshie (1992). "Characteristics of antisweet substances, sweet proteins, and sweetness‐inducing proteins". Critical Reviews in Food Science and Nutrition. 32 (3): 231–52. doi:10.1080/10408399209527598. PMID 1418601.