Gp41
Gp41 also known as glycoprotein 41 is a subunit of the envelope protein complex of retroviruses, including human immunodeficiency virus (HIV). Gp41 is a transmembrane protein that contains several sites within its ectodomain that are required for infection of host cells. As a result of its importance in host cell infection, it has also received much attention as a potential target for HIV vaccines.
| GP41 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Example crystal structures of HIV-1 envelope glycoprotein Gp41 | |||||||||
| Identifiers | |||||||||
| Symbol | GP41 | ||||||||
| Pfam | PF00517 | ||||||||
| InterPro | IPR000328 | ||||||||
| SCOPe | 2siv / SUPFAM | ||||||||
| |||||||||
Gene and post-translational modifications
Gp41 is coded with gp120 as one gp160 by the env gene of HIV. Gp160 is then extensively glycosylated and proteolytically cleaved by furin, a host cellular protease. The high glycosylation of the env coded glycoproteins allows them to escape the human body's immune system. In contrast to gp120, however, gp41 is less glycosylated and more conserved (less prone to genetic variations).[1] Once gp160 has been cleaved into its individual subunits, the subunits are then associated non-covalently on the surface of the viral envelope.
Structure
Gp41 and gp120, when non-covalently bound to each other, are referred to as the envelope spike complex and are formed as a heterotrimer of three gp41 and three gp120.[2] These complexes found on the surface of HIV are responsible for the attachment, fusion, and ultimately the infection of host cells. The structure is cage-like with a hollow center that inhibits antibody access. While gp120 sits on the surface of the viral envelope, gp41 is the transmembrane portion of the spike complex with a portion of the glycoprotein buried within the viral envelope at all times.[3]
Gp41 has three prominent regions within the sequence: the ectodomain, the transmembrane domain, and the cytoplasmic domain. The ectodomain, which comprises residues 511-684, can be further broken down into the fusion peptide region (residues 512-527), the helical N-terminal heptad repeat (NHR) and C-terminal heptad repeat (CHR).[3][4] In addition to these regions, there is also a loop region that contains disulfide bonds that stabilize the hairpin structure (the folded conformation of gp41) and a region called the membrane proximal external region (MPER) which contains kinks that are antigen target regions.[3][1] The fusion peptide region is normally buried or hidden by the non-covalent interactions between gp120 and gp41, at a point which looks torus-like. This prevents the fusion peptide from interacting with other regions that are not its intended target region.[2]
Function
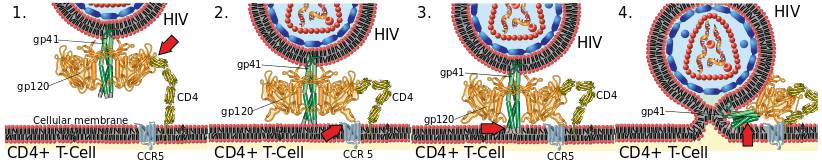
In a free virion, the fusion peptides at the amino termini of gp41 are buried within the envelope complex in an inactive non-fusogengic state that is stabilized by a non-covalent bond with gp120. Gp120 binds to a CD4 and a co-receptor (CCR5 or CXCR4), found on susceptible cells such as Helper T cells and macrophages.[5] As a result, a cascade of conformational changes occurs in the gp120 and gp41 proteins. These conformational changes start with gp120 that rearranges to expose the binding sites for the coreceptors mentioned above. The core of gp41 then folds into a six helical bundle (a coiled coil) structure exposing the previously hidden hydrophobic gp41 fusion peptides that are inserted in the host cell membrane allowing fusion to take place.[2] This fusion process is facilitated by the hairpin conformational structure.[6][7] The inner core of this conformation is 3 NHRs which have hydrophobic pockets that allow it to bind anti-parallel to specific residues on the CHR.[4][2] The activation process occurs readily, which suggests that the inactive state of gp41 is metastable and the conformational changes allow gp41 to achieve its more stable active state. Furthermore, these conformational changes are irreversible processes.[8]
As a drug target
The interaction of gp41 fusion peptides with the target cell causes a formation of an intermediate, pre-hairpin structure which bridges and fuses the viral and host membranes together. The pre-hairpin structure has a relatively long half-life which makes it a potential target for therapeutic intervention and inhibitory peptides.[9]
Enfuvirtide (also known as T-20) is a 36-residue alpha-peptide fusion inhibitor drug that binds to the pre-hairpin structure and prevents membrane fusion and HIV-1 entry to the cell. The vulnerability of this structure has initiated development towards a whole spectrum of fusion preventing drugs.[10][11] In developing these drugs, researchers face challenges because the conformation that allows for inhibition occurs very quickly and then rearranges.[12] Enfuviritide specifically has a low oral availability and is quickly processed and expelled by the body. Certain strains of HIV have also developed resistance to T-20. In order to circumvent the difficulties that come with using T-20, researchers have sought out peptide-based inhibitors.[3] A variety of naturally occurring molecules have also been shown to bind gp41 and prevent HIV-1 entry.[13]
The MPER is one region that has been studied as a potential target because of its ability to be recognized by broadly neutralizing antibodies (bNAbs), but it hasn't been a very good target because the immune response it elicits isn't very strong and because it is the portion of gp41 that enters the cell membrane (and it cannot be reached by antibodies then).[14] In addition to antigen binding regions on MPER kinks, there are other targets that could prove to be effective antigen binding regions, including the hydrophobic pockets of the NHR core that is formed following the conformational change in gp41 that creates the six-helix bundle.[1] These pockets could potentially serve as targets for small molecule inhibitors.[4] The fusion peptide on the N-terminus of the gp41 is also a potential target because it contains neutralizing antibody epitopes.[15] N36 and C34, or NHR- and CHR-based peptides (or short sequences of amino acids that mimic portions of gp41) can also act as effective antigens because of their high affinity binding. In addition to having a much higher affinity for binding when compared to its monomer, C34 also inhibits T-20 resistant HIV very well, which makes it a potentially good alternative to treatments involving enfuviritide.[12] Small-molecule inhibitors that are able to bind to two hydrophobic pockets at once have also been show to be 40-60 times more potent and have potential for further developments.[16] Most recently, the gp120-gp41 interface is being considered as a target for bNAbs.[1]
References
- Wibmer, Constantinos Kurt; Moore, Penny L.; Morris, Lynn (2015). "HIV broadly neutralizing antibody targets". Current Opinion in HIV and AIDS. 10 (3): 135–143. doi:10.1097/coh.0000000000000153. PMC 4437463. PMID 25760932.
- Mao, Youdong; Wang, Liping; Gu, Christopher; Herschhorn, Alon; Xiang, Shi-Hua; Haim, Hillel; Yang, Xinzhen; Sodroski, Joseph (2012). "Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer". Nature Structural & Molecular Biology. 19 (9): 893–899. doi:10.1038/nsmb.2351. PMC 3443289. PMID 22864288.
- Yi, Hyun A.; Fochtman, Brian C.; Rizzo, Robert C.; Jacobs, Amy (2016-01-01). "Inhibition of HIV Entry by Targeting the Envelope Transmembrane Subunit gp41". Current HIV Research. 14 (3): 283–294. doi:10.2174/1570162x14999160224103908. ISSN 1873-4251. PMC 4909398. PMID 26957202.
- Lu, Lu; Yu, Fei; Cai, Lifeng; Debnath, Asim; Jiang, Shibo (2015). "Development of Small-molecule HIV Entry Inhibitors Specifically Targeting gp120 or gp41". Current Topics in Medicinal Chemistry. 16 (10): 1074–1090. doi:10.2174/1568026615666150901114527. PMC 4775441. PMID 26324044.
- Chan DC, Kim PS (May 1998). "HIV entry and its inhibition". Cell. 93 (5): 681–4. doi:10.1016/S0092-8674(00)81430-0. PMID 9630213.
- Nomura, Wataru; Mizuguchi, Takaaki; Tamamura, Hirokazu (2016-07-01). "Multimerized HIV-gp41-derived peptides as fusion inhibitors and vaccines". Peptide Science. 106 (4): 622–628. doi:10.1002/bip.22782. ISSN 1097-0282. PMID 26583370.
- Buzon V, Natrajan G, Schibli D, Campelo F, Kozlov MM, Weissenhorn W (May 2010). "Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions". PLOS Pathogens. 6 (5): e1000880. doi:10.1371/journal.ppat.1000880. PMC 2865522. PMID 20463810.
- Munro, James B.; Mothes, Walther (2015-06-01). "Structure and Dynamics of the Native HIV-1 Env Trimer". Journal of Virology. 89 (11): 5752–5755. doi:10.1128/JVI.03187-14. ISSN 0022-538X. PMC 4442439. PMID 25762739.
- Lalezari JP, Henry K, O'Hearn M, Montaner JS, Piliero PJ, Trottier B, Walmsley S, Cohen C, Kuritzkes DR, Eron JJ, Chung J, DeMasi R, Donatacci L, Drobnes C, Delehanty J, Salgo M (May 2003). "Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America". The New England Journal of Medicine. 348 (22): 2175–85. doi:10.1056/NEJMoa035026. PMID 12637625.
- Root MJ, Steger HK (2004). "HIV-1 gp41 as a target for viral entry inhibition". Current Pharmaceutical Design. 10 (15): 1805–25. doi:10.2174/1381612043384448. PMID 15180542.
- Werner, Halina M; Horne, W Seth (2015-10-01). "Folding and function in α/β-peptides: targets and therapeutic applications". Current Opinion in Chemical Biology. Synthetic biology • Synthetic biomolecules. 28: 75–82. doi:10.1016/j.cbpa.2015.06.013. PMC 4624501. PMID 26136051.
- Yi HA, Fochtman BC, Rizzo RC, Jacobs A (2016-01-01). "Inhibition of HIV Entry by Targeting the Envelope Transmembrane Subunit gp41". Current HIV Research. 14 (3): 283–94. doi:10.2174/1570162x14999160224103908. PMC 4909398. PMID 26957202.
- Eade CR, Wood MP, Cole AM (January 2012). "Mechanisms and modifications of naturally occurring host defense peptides for anti-HIV microbicide development". Current HIV Research. 10 (1): 61–72. doi:10.2174/157016212799304580. PMC 4270272. PMID 22264047.
- Ghose, Chandrabali; Eugenis, Ioannis; Sun, Xingmin; Edwards, Adrianne N.; McBride, Shonna M.; Pride, David T.; Kelly, Ciarán P.; Ho, David D. (2016-02-03). "Immunogenicity and protective efficacy of recombinant Clostridium difficile flagellar protein FliC". Emerging Microbes & Infections. 5 (2): e8. doi:10.1038/emi.2016.8. PMC 4777929. PMID 26839147.
- Kong, Rui; Xu, Kai; Zhou, Tongqing; Acharya, Priyamvada; Lemmin, Thomas; Liu, Kevin; Ozorowski, Gabriel; Soto, Cinque; Taft, Justin D. (2016-05-13). "Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody". Science. 352 (6287): 828–833. Bibcode:2016Sci...352..828K. doi:10.1126/science.aae0474. ISSN 0036-8075. PMC 4917739. PMID 27174988.
- Sofiyev, Vladimir; Kaur, Hardeep; Snyder, Beth A.; Hogan, Priscilla A.; Ptak, Roger G.; Hwang, Peter; Gochin, Miriam (2017-01-01). "Enhanced potency of bivalent small molecule gp41 inhibitors". Bioorganic & Medicinal Chemistry. 25 (1): 408–420. doi:10.1016/j.bmc.2016.11.010. PMC 5260928. PMID 27908751.
External links
- gp41 Envelope Protein, HIV at the US National Library of Medicine Medical Subject Headings (MeSH)