Galileo thermometer
A Galileo thermometer (or Galilean thermometer) is a thermometer made of a sealed glass cylinder containing a clear liquid and several glass vessels of varying density. The individual floats rise or fall in proportion to their respective density and the density of the surrounding liquid as the temperature changes. It is named after Galileo Galilei because he discovered the principle on which this thermometer is based—that the density of a liquid changes in proportion to its temperature.

History
Although named after the 16th–17th-century physicist Galileo, the thermometer was not invented by him. (Galileo did invent a thermometer, called Galileo's air thermometer (more accurately termed a thermoscope), in or before 1603.)[1]
The instrument now known as a Galileo thermometer was invented by a group of academics and technicians known as the Accademia del Cimento of Florence,[2] who included Galileo's pupil, Torricelli and Torricelli's pupil Viviani.[3][4] Details of the thermometer were published in the Saggi di naturali esperienze fatte nell'Academia del Cimento sotto la protezione del Serenissimo Principe Leopoldo di Toscana e descritte dal segretario di essa Accademia (1666), the Academy's main publication. The English translation of this work (1684) describes the device ('The Fifth Thermometer') as 'slow and lazy', a description that is reflected in an alternative Italian name for the invention, the termometro lento (slow thermometer).[5] The outer vessel was filled with 'rectified spirits of wine' (a concentrated solution of ethanol in water); the weights of the glass bubbles were adjusted by grinding a small amount of glass from the sealed end; and a small air space was left at the top of the main vessel to allow 'for the Liquor to rarefie' [i.e. expand].
The device now called the Galileo thermometer was revived in the modern era by the Natural History Museum, London, which started selling a version in the 1990s.[6]
Operation
In the Galileo thermometer, the small glass bulbs are partly filled with different-coloured liquids. The composition of these liquids is not important for the functioning of the thermometer; they merely function as fixed weights and their colours are only for decoration. Once the hand-blown bulbs have been sealed, their effective densities are adjusted by means of the metal tags hanging from beneath them. Any expansion due to the temperature change of the coloured liquid and air gap inside the bulbs does not affect the operation of the thermometer, as these materials are sealed inside a glass bulb of fixed size. The clear liquid in which the bulbs are submerged is not water, but some organic compound (such as ethanol) the density of which varies with temperature more than water does. Temperature changes affect the density of the outer clear liquid and this causes the bulbs to rise or sink.[2]
Theory of operation

The Galilean thermometer works on the principle of buoyancy. Buoyancy determines whether objects float or sink in a liquid, and is responsible for the fact that even boats made of steel float in water while a solid bar of steel sinks.
The only factor that determines whether a large object rises or falls in a particular liquid is the object's density relative to the density of the liquid. If the object is denser than the liquid, it sinks to the bottom, as it is heavier than the liquid it displaces. If it is less dense, it floats on the water, and will be partially submerged until the weight of the displaced liquid becomes equal to the object's weight.
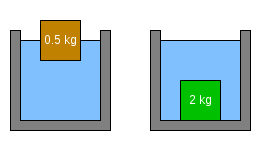
Suppose there are two objects, each with a volume of 1 L (0.22 imp gal; 0.26 US gal). The mass of water displaced by an object of this size is 1 kg (2.2 lb). The brown object on the left is floating because the mass of water it would displace if completely submerged (1 kg (2.2 lb)) is greater than the mass of the object. It floats half submerged because that is the point where the mass of the water displaced (0.5 kg (1.1 lb)) is equal to the mass of the object. The green object on the right has sunk because the mass of water it is displacing (1 kg (2.2 lb)) is less than the object's mass (2 kg (4.4 lb)).
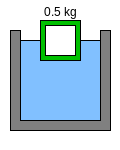
All objects made of the green material above will sink. In Figure 2, the interior of the green object has been hollowed out. The total mass of the object is now 0.5 kg (1.1 lb), yet its volume remains the same, so it floats halfway out of the water like the brown object in Figure 1.
In the examples above, the liquid in which the objects have been floating is assumed to be water. Water has a density of 1 kilogram per litre (8.3 lb/US gal), which means that the mass of water displaced by any of the above objects when fully submerged, is 1 kg (2.2 lb).
Galileo discovered that the density of a liquid is a function of its temperature. This is the key to how the Galileo thermometer works: as the temperature of most liquids increases, their density decreases.
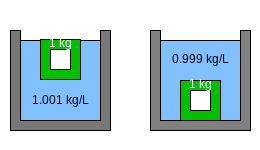
Figure 3 shows a 1 kg (2.2 lb) hollow object made of the green material. In the left-hand container, the density of the liquid is 1.001 kg/L (8.35 lb/US gal). Since the object weighs less than the water it displaces, it floats. In the right-hand container, the density of the liquid is 0.999 kg/L (8.34 lb/US gal). Since the object weighs more than the mass of the water it displaces, it sinks. This shows that very small changes in the density of the liquid can easily cause an almost-floating object to sink.
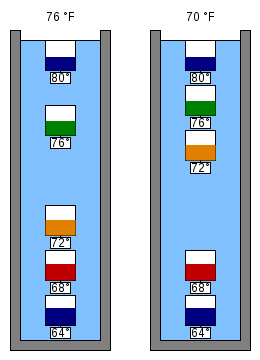
Figure 4 shows a schematic representation of a Galileo thermometer at two different temperatures.
In some models, if there are some bulbs at the top (Figure 4, left) and some at the bottom, but one floating in the gap, then the one floating in the gap (green 76 °F (24 °C)) tells the temperature. If there is no bulb in the gap (Figure 4, right) then the average of the values of the bulb above and below the gap gives the approximate temperature. In other models, the lowest floating bulb gives the approximate temperature.[7]
The weight and size of each bulb is adjusted so they do not jam together, either by being at least half the size of the tube diameter to retain their stacking order or, as an alternative, less than half the tube diameter to freely pass each other in the tubes.
See also
Notes
| Wikimedia Commons has media related to Galileo thermometer. |
- Aleksandr Khristoforovich Khrgian, Meteorology: a historical survey, Volume 1, Israel Program for Scientific Translations, 1970, p. 25
- Loyson, Peter (2012). "Galilean Thermometer Not So Galilean". Journal of Chemical Education. 89 (9): 1095–1096. Bibcode:2012JChEd..89.1095L. doi:10.1021/ed200793g.
- Fretwell, Mattie Bell (February 1937). "The Development if the Thermometer". Mathematics Teacher. 30 (2): 80–83. JSTOR 27952013.
- A. Frova & M. Marenzana, Thus spoke Galileo, p.348, accessed on Google Books 2012-06-14, itself based on R. Caverni, Storia del metodo sperimentale in Italia, Vol. 2, Florence, 1895
- José Montesinos, Carlos Solís Santos [eds], Largo campo di filosofare: Eurosymposium Galileo 2001, Fundación Canaria Orotava, 2001
- Daily Mirror, 28 January 1994, p. 28
- How to read a Galilean Thermometer
