Freundlich equation
The Freundlich equation or Freundlich adsorption isotherm, an adsorption isotherm, is an empirical relation between the concentration of a solute on the surface of an adsorbent to the concentration of the solute in the liquid with which it is in contact. In 1909, Herbert Freundlich gave an expression representing the isothermal variation of adsorption of a quantity of gas adsorbed by unit mass of solid adsorbent with pressure.[1] This equation is known as Freundlich adsorption isotherm or Freundlich adsorption equation. As this relationship is entirely empirical, in the case where adsorption behavior can be properly fit by isotherms with a theoretical basis, it is usually appropriate to use such isotherms instead (see for example the Langmuir and BET adsorption theories). The Freundlich equation is also derived (non-empirically) by attributing the change in the equilibrium constant of the binding process to the heterogeneity of the surface and the variation in the heat of adsorption[2]..
Freundlich adsorption isotherm
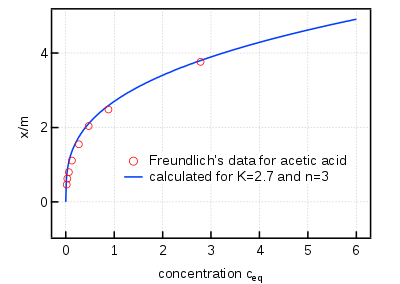
The Freundlich adsorption isotherm is mathematically expressed as
It is also written as
or
It is also written as
where
- x = mass of adsorbate
- m = mass of adsorbent
- p = Equilibrium pressure of adsorbate
- c = Equilibrium concentration of adsorbate in solution.
K and n are constants for a given adsorbate and adsorbent at a particular temperature.
At high pressure 1/n = 0, hence extent of adsorption becomes independent of pressure.
It is used in cases where the actual identity of the solute is not known, such as adsorption of colored material from sugar, vegetable oil etc.
The Freundlich equation is unique; consequently, if the data fit the equation, it is only likely, but not proved, that the surface is heterogeneous. The heterogeneity of the surface can be confirmed with calorimetery. Homogeneous surfaces (or heterogeneous surfaces that exhibit homogeneous adsorption (single site)) have a constant of adsorption. On the other hand, heterogenous adsorption (multi-site) have a variable of adsorption depending on the percent of sites occupied. When the adsorbate pressure (or concentration) are low, high energy sites will be occupied; and as the pressure (or concentration) increases, the lesser energy sites will be occupied resulting in a lower of adsorption[4].
Limitation of Freundlich adsorption isotherm
Experimentally it was determined that extent of gas adsorption varies directly with pressure, and then it directly varies with pressure raised to the power 1/n until saturation pressure Ps is reached. Beyond that point, the rate of adsorption saturates even after applying higher pressure. Thus, the Freundlich adsorption isotherm fails at higher pressure.
See also
References
- Freundlich, Herbert. Kapillarchemie, eine Darstellung der Chemie der Kolloide und verwandter Gebiete. Akademische Verlagsgesellschaft, 1909.
- Adamson, A.W (1997). physical chemistry of surfaces. p. 393.
- Freundlich, Herbert. " Über die Adsorption in Lösungen." Zeitschrift für Physikalische Chemie - Stöchiometrie und Verwandschaftslehre (1907): Volume 57, Issue 4, pages 385-470.
- Adamson, A.W (1997). physical chemistry of surfaces. p. 699.
Further reading
- Jaroniec, M. (1975). "Adsorption on heterogeneous surfaces: The exponential equation for the overall adsorption isotherm". Surface Science. 50 (2): 553–564. Bibcode:1975SurSc..50..553J. doi:10.1016/0039-6028(75)90044-8.
- Levan, M. Douglas; Vermeulen, Theodore (1981). "LeVan, M. Douglas, and Theodore Vermeulen. "Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions." The Journal of Physical Chemistry 85.22 (1981): 3247-3250". The Journal of Physical Chemistry. 85 (22): 3247–3250. doi:10.1021/j150622a009.
- "Freundlich Equation". Archived from the original on 3 March 2016.
External links
- "Freundlich equation solver".
- "Freundlich Adsorption Isotherm". Archived from the original on 2 March 2012.