Fatal insomnia
Fatal insomnia is a rare disorder that results in trouble sleeping.[2] The problems sleeping typically start out gradually and worsen over time.[3] Other symptoms may include speech problems, coordination problems, and dementia.[4][5] It results in death within a few months to a few years.[2]
| Fatal insomnia | |
|---|---|
 | |
| Cranial imaging of an FFI patient. In the MRI, there are abnormal signals in the bilateral frontoparietal subcortical area. MRA showed smaller distal branches of cerebral arteries. | |
| Specialty | Psychiatry, Sleep medicine, Neuropathology |
| Symptoms | Progressive insomnia leading to dementia and death. |
| Usual onset | 32–62 years[1] |
| Types | Fatal familial insomnia, sporadic fatal insomnia[2] |
| Causes | Genetic mutation, sporadic form very rare |
| Risk factors | Family history |
| Diagnostic method | Sleep study, PET scan, genetic testing[1] |
| Differential diagnosis | Creutzfeldt–Jakob disease, Alzheimer’s disease, frontotemporal dementia[3] |
| Treatment | Supportive care[2] |
| Prognosis | Life expectancy 7 months to 6 years[2] |
| Frequency | Rare[3] |
It is a prion disease of the brain.[2] It is usually caused by a mutation to the gene encoding protein PrPC.[2] It has two forms: fatal familial insomnia (FFI), which is autosomal dominant and sporadic fatal insomnia (sFI) which is due to a noninherited mutation. Diagnosis is based on a sleep study, PET scan, and genetic testing.[1]
Fatal insomnia has no known cure and involves progressively worsening insomnia, which leads to hallucinations, delirium, confusional states like that of dementia, and eventually death.[6] The average survival time from onset of symptoms is 18 months.[6] The first recorded case was an Italian man, who died in Venice in 1765.[7]
Signs and symptoms
The disease has four stages:[8]
- The person has increasing insomnia, resulting in panic attacks, paranoia, and phobias. This stage lasts for about four months.
- Hallucinations and panic attacks become noticeable, continuing for about five months.
- Complete inability to sleep is followed by rapid loss of weight. This lasts for about three months.
- Dementia, during which the person becomes unresponsive or mute over the course of six months, is the final stage of the disease, after which death follows.
Other symptoms include profuse sweating, pinpoint pupils, the sudden entrance into menopause for women and impotence for men, neck stiffness, and elevation of blood pressure and heart rate. Constipation is common as well. As the disease progresses, the person becomes stuck in a state of pre-sleep limbo, or hypnagogia, which is the state just before sleep in healthy individuals. During these stages, people commonly and repeatedly move their limbs as if dreaming.[9]
The age of onset is variable, ranging from 18 to 60 years, with an average of 50.[10] The disease can be detected prior to onset by genetic testing.[11] Death usually occurs between 7–36 months from onset. The presentation of the disease varies considerably from person to person, even among people within the same family.
Cause
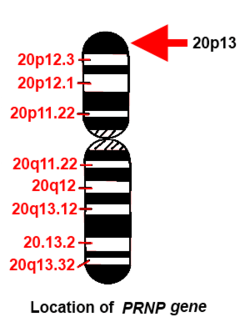
The gene PRNP that provides instructions for making the prion protein PrPC is located on the short (p) arm of chromosome 20 at position p13.[12] Both people with FFI and those with familial Creutzfeldt–Jakob disease (fCJD) carry a mutation at codon 178 of the prion protein gene. FFI is also invariably linked to the presence of the methionine codon at position 129 of the mutant allele, whereas fCJD is linked to the presence of the valine codon at that position. "The disease is where there is a change of amino acid at position 178 when an asparagine (N) is found instead of the normal aspartic acid (D). This has to be accompanied with a methionine at position 129."[13]
Pathophysiology
In itself, the presence of prions causes reduced glucose use by the thalamus and a mild hypo-metabolism of the cingulate cortex. The extent of this symptom varies between two variations of the disease, these being those presenting methionine homozygotes at codon 129 and methionine/valine heterozygotes being the most severe in the later one.[14] Given the relationship between the involvement of the thalamus in regulating sleep and alertness, a causal relationship can be drawn, and is often mentioned as the cause.
Diagnosis
Diagnosis is suspected based on symptoms.[1] Further work up often include a sleep study and PET scan.[1] Confirmation of the familial form is by genetic testing.[1]
Differential diagnosis
Other diseases involving the mammalian prion protein are known.[15] Some are transmissible (TSEs, including FFI) such as kuru, bovine spongiform encephalopathy (BSE, also known as "mad cow disease") in cattle, and chronic wasting disease in American deer and American elk in some areas of the United States and Canada, as well as Creutzfeldt–Jakob disease (CJD). Until recently, prion diseases were only thought to be transmissible by direct contact with infected tissue, such as from eating infected tissue, transfusion, or transplantation; research suggests that prions can be transmitted by aerosols, but that the general public is not at risk of airborne infection.[16]
Treatments
Treatment involves supportive care.[2] Sleeping pills, including barbiturates, have not been found to be helpful; on the contrary, they have been suggested to worsen the symptoms.[17]
Prognosis
The disease is invariably fatal.[6][2] Life expectancy ranges from seven months to six years,[2] with an average of 18 months.[6]
Epidemiology

In 1998, 40 families were known to carry the gene for FFI globally: eight German, five Italian, four American, two French, two Australian, two British, one Japanese, and one Austrian.[18] In the Basque Country, Spain, 16 family cases of the 178N mutation were seen between 1993 and 2005 related to two families with a common ancestor in the 18th century.[19] In 2011, another family was added to the list when researchers found the first man in the Netherlands with FFI. While he had lived in the Netherlands for 19 years, he was of Egyptian descent.[20] Other prion diseases are similar to FFI and could be related, but are missing the D178N gene mutation.[9]
As of 2016, 24 cases of sporadic fatal insomnia have been diagnosed.[1] Unlike in FFI, sFI sufferers do not have the D178N mutation in the PRNP-prion gene; they all have a different mutation in the same gene causing methionine homozygosity at codon 129.[21][22]
Silvano, 1983, Bologna, Italy
In late 1983, Italian neurologist/sleep expert Dr. Ignazio Roiter received a patient at the University of Bologna hospital's sleep institute. The man, known only as Silvano, decided in a rare moment of consciousness to be recorded for future studies and to donate his brain for research in hopes of finding a cure for future victims.[23]
Unnamed patient of Schenkein and Montagna, 2001
One person was able to exceed the average survival time by nearly one year with various strategies, including vitamin therapy and meditation, using different stimulants and hypnotics, and even complete sensory deprivation in an attempt to induce sleep at night and increase alertness during the day. He managed to write a book and drive hundreds of miles in this time, but nonetheless, over the course of his trials, the person succumbed to the classic four-stage progression of the illness.[24][23]
Egyptian man, 2011, Netherlands

In 2011, the first reported case in the Netherlands was of a 57 year-old man of Egyptian descent. The man came in with symptoms of double vision and progressive memory loss, and his family also noted he had recently become disoriented, paranoid, and confused. While he tended to fall asleep during random daily activities, he experienced vivid dreams and random muscular jerks during normal slow-wave sleep. After four months of these symptoms, he started having convulsions in the hands, trunk, and lower limbs while awake. The person died at age 58, seven months after the onset of symptoms. An autopsy revealed mild atrophy of the frontal cortex and moderate atrophy of the thalamus. The latter is one of the most common signs of FFI.[20]
Popular culture
Charlie Huston's 2010 novel Sleepless concerns an epidemic of a fatal insomnia that is frequently compared to FFI by characters in the story.
In Something's Killing Me with BD Wong, November 2017 (season one, episode five), "Family Curse", FFI is the topic.[25]
Nancy Kress's novelette Pathways concerns research into FFI.[26]
FFI is a major plot element and is described in detail in the Inspector Lewis episode “Falling Darkness”.
Research
Still with unclear benefit in humans, a number of treatments have had tentative success in slowing disease progression in animal models, including pentosan polysulfate, mepacrine, and amphotericin B.[1] As of 2016, a study investigating doxycycline is being carried out.[1][27]
In 2009, a mouse model was made for FFI. These mice expressed a humanized version of the PrP protein that also contains the D178N FFI mutation.[28] These mice appear to have progressively fewer and shorter periods of uninterrupted sleep, damage in the thalamus, and early deaths, similar to humans with FFI.
The Prion Alliance was established by husband and wife duo Eric Minikel and Sonia Vallabh after Vallabh was diagnosed with the fatal disease.[29] They conduct research at the Broad Institute to develop therapeutics for human prion diseases. Other research interests involve identifying biomarkers to track the progression of prion disease in living people.[30][31]
References
- "Fatal familial insomnia". Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. Retrieved 17 May 2019.
- "Fatal Insomnia - Neurologic Disorders". Merck Manuals Professional Edition. Retrieved 17 May 2019.
- "Fatal Familial Insomnia". NORD (National Organization for Rare Disorders). Retrieved 17 May 2019.
- "What is fatal familial insomnia?". Healthline. 26 January 2018. Retrieved 4 May 2018.
- "Fatal Insomnia". Merck Manual. Retrieved 4 May 2018.
- Schenkein J, Montagna P (2006). "Self management of fatal familial insomnia. Part 1: what is FFI?". MedGenMed. 8 (3): 65. PMC 1781306. PMID 17406188.
- Max, D. T. (2007). The Family that Couldn't Sleep: A medical mystery. New York: Random House Trade Paperbacks. p. 4.
- Turner, Rebecca. "Dying To Sleep: Fatal Familial Insomnia (FFI)". www.world-of-lucid-dreaming.com. Retrieved 22 March 2018.
- Cortelli, Pietro; Gambetti, Pierluigi; Montagna, Pasquale & Lugaresi, Elio (1999). "Fatal familial insomnia: clinical features and molecular genetics". Journal of Sleep Research. 8: 23–29. doi:10.1046/j.1365-2869.1999.00005.x.
- "Episode 25: Fatal Insomnia". Obscura: A True Crime Podcast.
- Max, D.T. (May 2010). "The Secret of Sleep". National Geographic Magazine. p. 74.
- Reference, Genetics Home h. "PRNP gene". Genetics Home Reference. Retrieved 22 March 2018.
- "Zalan Khan; Pradeep C. Bollu, Fatal Familial Insomnia". NCBI Bookshelf.
- Cortelli., P. (1 July 1997). "Cerebral metabolism in fatal familial insomnia: Relation to duration, neuropathology, and distribution of protease-resistant prion protein". Neurology. 49 (1). doi:10.1212/WNL.49.1.126. Retrieved 1 November 2019.
- Panegyres, Peter; Burchell, Jennifer T. (2016). "Prion diseases: Immunotargets and therapy". ImmunoTargets and Therapy. 5: 57–68. doi:10.2147/ITT.S64795. ISSN 2253-1556. PMC 4970640. PMID 27529062.
- Mosher, Dave (13 January 2011). "Airborne prions make for 100 percent lethal whiff". Wired. Retrieved 20 May 2011.
- Turner, Rebecca. "The man who never slept: Michael Corke". World of Lucid Dreaming. Retrieved 20 May 2011.
- Montagna P, Gambetti P, Cortelli P, Lugaresi E (2003). "Familial and sporadic fatal insomnia". Lancet Neurol. 2 (3): 167–76. doi:10.1016/S1474-4422(03)00323-5. PMID 12849238.
- Zarranz JJ, Arteagoitia JM, Atarés B, Rodríguez-Martínez AB, Martínez-de-Pancorbo M, et al. (2007). "Las encefalopatias espongiformes o enfermedades por priones en el País Vasco". GacMedBilbao. 104 (2): 64–69. doi:10.1016/S0304-4858(07)74572-9. PMID 10371520.
- Jansen, C.; Parchi, P.; Jelles, B.; Gouw, A. A.; Beunders, G.; van Spaendonk, R. M. L.; van de Kamp, J. M.; Lemstra, A. W.; Capellari, S.; Rozemuller, A. J. M. (13 July 2011). "The first case of fatal familial insomnia (FFI) in the Netherlands: a patient from Egyptian descent with concurrent four repeat tau deposits". Neuropathology and Applied Neurobiology. 37 (5): 549–553. doi:10.1111/j.1365-2990.2010.01126.x. PMID 20874730.
- Mehta LR, Huddleston BJ, Skalabrin EJ, et al. (July 2008). "Sporadic fatal insomnia masquerading as a paraneoplastic cerebellar syndrome". Arch. Neurol. 65 (7): 971–973. doi:10.1001/archneur.65.7.971. PMID 18625868.
- Moody KM, Schonberger LB, Maddox RA, Zou WQ, Cracco L, Cali I (2011). "Sporadic fatal insomnia in a young woman: A diagnostic challenge". Case report. BMC Neurol. 11: 136. doi:10.1186/1471-2377-11-136. PMC 3214133. PMID 22040318.
- Schenkein J, Montagna P (2006). "Part 2: Case report". MedGenMed: Medscape General Medicine. Self-management of fatal familial insomnia. 8 (3): 66. PMC 1781276. PMID 17406189.
- "Dying without sleep: Insomnia and its implications". triplehelixblog.com. 16 June 2011. Retrieved 22 March 2018.
- "Something's Killing Me". TVGuide.com. Retrieved 22 March 2018.
- "Pathways". isfdb.org. Retrieved 9 December 2018.
- Forlonia, Gianluigi; Tettamantia, Mauro; Luccaa, Ugo; Albanesea, Yasmin; Quaglioa, Elena; Chiesaa, Roberto; Erbettab, Alessandra; Villanib, Flavio; Redaellib, Veronica; Tagliavinib, Fabrizio; Artusoc, Vladimiro; Roiterc, Ignazio (21 May 2015). "Preventive study in subjects at risk of fatal familial insomnia: Innovative approach to rare diseases". Prion. 9 (2): 75–79. doi:10.1080/19336896.2015.1027857. PMC 4601344. PMID 25996399.
- Jackson W, et al. (2009). "Spontaneous beneration of prion infectivity in fatal familial insomnia Knockin mice". Neuron. 63 (4): 438–450. doi:10.1016/j.neuron.2009.07.026. PMC 2775465. PMID 19709627.
- Clancy, Kelly (15 January 2019). "One Couple's Tireless Crusade to Stop a Genetic Killer". Wired. ISSN 1059-1028. Retrieved 21 January 2019.
- "Sonia Vallabh". Broad Institute. 20 August 2015. Retrieved 21 January 2019.
- "Prion Alliance". www.prionalliance.org. Retrieved 21 January 2019.
External links
| Classification |
|---|