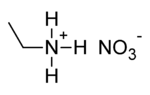
Ethylammonium nitrate
Ethylammonium nitrate or ethylamine nitrate[2] (EAN) is a salt with formula C
2H
8N
2O
3 or (C
2H
5)NH+
3·NO−
3. It is an odorless and colorless to slightly yellowish liquid with a melting point of 12 °C.[3] This compound was described by Paul Walden in 1914,[4][5] and is believed to be the earliest reported example of a room-temperature ionic liquid.[6]
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.218.244 | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties[1] | |||
| C 2NH 8NO 3 | |||
| Molar mass | 108.0965 g mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.261 g/ml | ||
| Melting point | 12 °C (54 °F; 285 K) | ||
| Boiling point | 240 °C (464 °F; 513 K) | ||
| Thermochemistry | |||
Heat capacity (C) |
206 J K−1 mol−1 | ||
| Hazards | |||
| Main hazards | Irritant | ||
| Safety data sheet | |||
| R-phrases (outdated) | R36/37/38 | ||
| S-phrases (outdated) | S24/25 S37/39 | ||
| Related compounds | |||
Other cations |
Methylammonium nitrate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis and properties
Ethylammonium nitrate can be produced by heating ethyl nitrate with an alcoholic solution of ammonia[7] or by reacting ethylamine with concentrated nitric acid.[5] It has a relatively low viscosity of 0.28 poise or 0.028 Pa·s at 25 °C and therefore a high electrical conductivity of about 20 mS·cm−1 at 25 °C. It boils at 240 °C and decomposes at about 250 °C.[1] Its density at 20 °C is 1.261 g/cm3.[8]
The ethylammonium ion (C
2H
5)NH+
3 has three easily detachable protons which are tetrahedrally arranged around the central nitrogen atom, whereas the configuration of the NO−
3 anion is planar. Despite the structural differences, EAN shares many properties with water, such as micelle formation, aggregation of hydrocarbons, negative enthalpy and entropy of dissolution of gases, etc. Similar to water, EAN can form three-dimensional hydrogen bonding networks.[9]
Applications
Ethylammonium nitrate is used as an electrically conductive solvent in electrochemistry and as a protein crystallization agent.[10][11] It has a positive effect on the refolding of denaturated lysozyme, with the refolding yield of about 90%. The refolding action was explained as follows: The ethyl group of ethylammonium nitrate interacts with the hydrophobic part of the protein and thereby protects it from intermolecular association, whereas the charged part of EAN stabilizes the electrostatic interactions.[12]
References
- Ionic liquids & ionic liquid acids with high temperature stability for fuel cell and other high temperature applications, method of making and cell employing same United States Patent Application 20070026295, Google patents link
- Wagaman, Kerry L Liquid monopropellant United States Patent 6001197, Publication Date 12/14/1999
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. (2004). "Room temperature ionic liquids and their mixtures—a review". Fluid Phase Equilibria. 219: 93–98. doi:10.1016/j.fluid.2004.02.003.
- P. Walden (1914). Chem. Zentralbl. 85: 1800–1801. Missing or empty
|title=(help) - P. Walden (1914). "Ueber die Molekulargrösse und elektrische Leitfähigkeit einiger geschmolzenen Salze". Bull. Acad. Imper. Sci. St. Pétersbourg. 6. 8: 405–422. External link in
|title=(help) - Mihkel Koel (2008). Ionic Liquids in Chemical Analysis. CRC Press. p. xxvii. ISBN 978-1-4200-4646-5.
- Rudolph Fittig (2008). Wohler's Outlines of Organic Chemistry. Read Books. p. 56. ISBN 978-1-4097-9043-3.
- "Safety Data Sheet" (PDF). Carlroth. Retrieved 19 September 2016.
- Allen, Martin; Evans, D. Fennell; Lumry, Rufus (1985). "Thermodynamic properties of the ethylammonium nitrate + water system: Partial molar volumes, heat capacities, and expansivities". Journal of Solution Chemistry. 14 (8): 549. doi:10.1007/BF00649520.
- Garlitz, Jennifer A.; Summers, Catherine A.; Flowers, Robert A.; Borgstahl, Gloria E. O. (1999). "Ethylammonium nitrate: a protein crystallization reagent". Acta Crystallographica D. 55 (12): 2037–8. doi:10.1107/S0907444999011774. PMID 10666583.
- M. Riad Manaa (2005). Chemistry at extreme conditions. Elsevier. p. 441. ISBN 0-444-51766-9.
- Jochen Decker, Udo Reischl (2004). Molecular diagnosis of infectious diseases. Humana Press. p. 247. ISBN 1-58829-221-5.