Ethyl nitrate
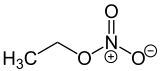
Ethyl nitrate is the ethyl ester of nitric acid and has the chemical formula C2H5NO3. It is a colourless, volatile, explosive, and highly flammable liquid. It is used in organic synthesis and as an intermediate in the preparation of some drugs, dyes, and perfumes.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ethyl nitrate | |
| Other names
Nitric acid ethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.913 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5NO3 | |
| Molar mass | 91.07 g/mol |
| Appearance | colorless liquid |
| Density | 1.10g/cm3 |
| Melting point | −102 °C (−152 °F; 171 K) |
| Boiling point | 87.5 °C (189.5 °F; 360.6 K) |
| soluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | −37 °C; −34 °F; 236 K |
| Explosive limits | 4.1%-50% |
| Related compounds | |
Related Alkyl nitrates |
Methyl nitrate Ethylene glycol dinitrate Isopropyl nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl nitrate is found in the atmosphere, where it can react with other gases to form smog. Originally thought to be a pollutant, formed mainly by the combustion of fossil fuels, recent analysis of ocean water samples reveal that in places where cool water rises from the deep, the water is saturated with alkyl nitrates, likely formed by natural processes.[2]
Preparation
Ethyl nitrate has been prepared by bubbling gaseous nitryl fluoride through ethanol at −10 °C.[3] The reaction was subsequently studied in detail.[4][5]
Ethyl nitrate can be prepared by nitrating ethanol with fuming nitric acid or a mixture of concentrated sulfuric and nitric acids. Further purifying by distillation carries a risk of explosion.[6]
References
- 1921-, Schofield, Kenneth (1980). Aromatic nitration. Cambridge: Cambridge University Press. p. 94. ISBN 9780521233620. OCLC 6357479.CS1 maint: numeric names: authors list (link)
- S. Perkins (August 12, 2002). "Ocean yields gases that had seemed humanmade". Science News (only available to subscribers).
- G. Hetherington and R. L. Robinson (1954). "Nitryl fluoride as a nitrating agent". J. Chem. Soc.: 3512. doi:10.1039/JR9540003512.
- B. S. Fedorov and L. T. Eremenko (1997). "Nitration of alcohols by nitryl fluoride". Russian Chemical Bulletin. 46 (5): 1022–1023. doi:10.1007/BF02496138.
- Explosives, 6th Edition, R. Meyer, J. Kohler, A. Homburg; page 125
- Cohen, Julius B. (Julius Berend) (1920). Theoretical organic chemistry. University of California Libraries. London, Macmillan. p. 189.
