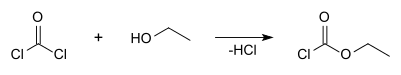Ethyl chloroformate
Ethyl chloroformate is the ethyl ester of chloroformic acid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group[2] and for the formation of carboxylic anhydrides.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl carbonochloridate | |
| Other names
Chloroformic acid ethyl ester Cathyl chloride Ethyl chlorocarbonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.981 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H5ClO2 | |
| Molar mass | 108.52 g/mol |
| Appearance | Clear liquid |
| Density | 1.1403 g/cm3 |
| Boiling point | 95 °C (203 °F; 368 K) |
| Decomposes | |
| Hazards | |
| Main hazards | Corrosive Flammable |
| NFPA 704 (fire diamond) | |
| Flash point | 61 °C (142 °F; 334 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Safety
Ethyl chloroformate is a highly toxic substance. It causes severe burns when comes in contact with eyes and/or skin, can be fatal if swallowed or inhaled.[3]
gollark: Though given that many people apparently can't even run the uninstaller thing most potatOS users probably can't manage bypassing even that.
gollark: When I say "XOR encryption" I mean "XOR everything with one fixed byte", i.e. basically not encryption.
gollark: Hi.
gollark: It's equal to 130 iterations of rot1.
gollark: PotatOS actually integrates advanced 5rot26 encryption already.
References
- Merck Index, 11th Edition, 3742.
- Protective Groups in Organic Synthesis, Third Edition, Theodora W. Greene and Peter G. M. Wuts, pages 504-506, ISBN 0-471-16019-9
- PubChem. "Ethyl chloroformate". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-09-04.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.