Effective nuclear charge
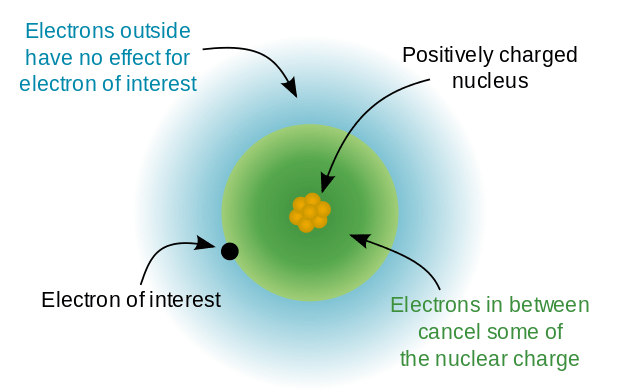
The effective nuclear charge (often symbolized as or ) is the net positive charge experienced by an electron in a polyelectronic atom. The term "effective" is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner-layer electrons. The effective nuclear charge experienced by the electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom. Most of the physical and chemical properties of the elements can be explained on the basis of electronic configuration. Consider the behavior of ionization energies in the periodic table. It is known that the magnitude of ionization potential depends upon the following factors:
(a) Size of atom;
(b) The nuclear charge;
(c) The screening effect of the inner shells, and;
(d) The extent to which the outermost electron penetrates into the charge cloud set up by the inner lying electron.
Calculations
In an atom with one electron, that electron experiences the full charge of the positive nucleus. In this case, the effective nuclear charge can be calculated by Coulomb's law.
However, in an atom with many electrons, the outer electrons are simultaneously attracted to the positive nucleus and repelled by the negatively charged electrons. The effective nuclear charge on such an electron is given by the following equation:
where
- Z is the number of protons in the nucleus (atomic number), and
- S is the shielding constant.
S can be found by the systematic application of various rule sets, the simplest of which is known as "Slater's rules" (named after John C. Slater). Douglas Hartree defined the effective Z of a Hartree–Fock orbital to be:
where
Values
Updated effective nuclear charge values were provided by Clementi et al. in 1963 and 1967.[1][2] In their work, screening constants were optimized to produce effective nuclear charge values that agree with SCF calculations. Though useful as a predictive model, the resulting screening constants contain little chemical insight as a qualitative model of atomic structure.
| H | He | |||||||||||||||||
| Z | 1 | 2 | ||||||||||||||||
| 1s | 1.000 | 1.688 | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Z | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||
| 1s | 2.691 | 3.685 | 4.680 | 5.673 | 6.665 | 7.658 | 8.650 | 9.642 | ||||||||||
| 2s | 1.279 | 1.912 | 2.576 | 3.217 | 3.847 | 4.492 | 5.128 | 5.758 | ||||||||||
| 2p | 2.421 | 3.136 | 3.834 | 4.453 | 5.100 | 5.758 | ||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Z | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||
| 1s | 10.626 | 11.609 | 12.591 | 13.575 | 14.558 | 15.541 | 16.524 | 17.508 | ||||||||||
| 2s | 6.571 | 7.392 | 8.214 | 9.020 | 9.825 | 10.629 | 11.430 | 12.230 | ||||||||||
| 2p | 6.802 | 7.826 | 8.963 | 9.945 | 10.961 | 11.977 | 12.993 | 14.008 | ||||||||||
| 3s | 2.507 | 3.308 | 4.117 | 4.903 | 5.642 | 6.367 | 7.068 | 7.757 | ||||||||||
| 3p | 4.066 | 4.285 | 4.886 | 5.482 | 6.116 | 6.764 | ||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Z | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
| 1s | 18.490 | 19.473 | 20.457 | 21.441 | 22.426 | 23.414 | 24.396 | 25.381 | 26.367 | 27.353 | 28.339 | 29.325 | 30.309 | 31.294 | 32.278 | 33.262 | 34.247 | 35.232 |
| 2s | 13.006 | 13.776 | 14.574 | 15.377 | 16.181 | 16.984 | 17.794 | 18.599 | 19.405 | 20.213 | 21.020 | 21.828 | 22.599 | 23.365 | 24.127 | 24.888 | 25.643 | 26.398 |
| 2p | 15.027 | 16.041 | 17.055 | 18.065 | 19.073 | 20.075 | 21.084 | 22.089 | 23.092 | 24.095 | 25.097 | 26.098 | 27.091 | 28.082 | 29.074 | 30.065 | 31.056 | 32.047 |
| 3s | 8.680 | 9.602 | 10.340 | 11.033 | 11.709 | 12.368 | 13.018 | 13.676 | 14.322 | 14.961 | 15.594 | 16.219 | 16.996 | 17.790 | 18.596 | 19.403 | 20.219 | 21.033 |
| 3p | 7.726 | 8.658 | 9.406 | 10.104 | 10.785 | 11.466 | 12.109 | 12.778 | 13.435 | 14.085 | 14.731 | 15.369 | 16.204 | 17.014 | 17.850 | 18.705 | 19.571 | 20.434 |
| 4s | 3.495 | 4.398 | 4.632 | 4.817 | 4.981 | 5.133 | 5.283 | 5.434 | 5.576 | 5.711 | 5.842 | 5.965 | 7.067 | 8.044 | 8.944 | 9.758 | 10.553 | 11.316 |
| 3d | 7.120 | 8.141 | 8.983 | 9.757 | 10.528 | 11.180 | 11.855 | 12.530 | 13.201 | 13.878 | 15.093 | 16.251 | 17.378 | 18.477 | 19.559 | 20.626 | ||
| 4p | 6.222 | 6.780 | 7.449 | 8.287 | 9.028 | 9.338 | ||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Z | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 |
| 1s | 36.208 | 37.191 | 38.176 | 39.159 | 40.142 | 41.126 | 42.109 | 43.092 | 44.076 | 45.059 | 46.042 | 47.026 | 48.010 | 48.992 | 49.974 | 50.957 | 51.939 | 52.922 |
| 2s | 27.157 | 27.902 | 28.622 | 29.374 | 30.125 | 30.877 | 31.628 | 32.380 | 33.155 | 33.883 | 34.634 | 35.386 | 36.124 | 36.859 | 37.595 | 38.331 | 39.067 | 39.803 |
| 2p | 33.039 | 34.030 | 35.003 | 35.993 | 36.982 | 37.972 | 38.941 | 39.951 | 40.940 | 41.930 | 42.919 | 43.909 | 44.898 | 45.885 | 46.873 | 47.860 | 48.847 | 49.835 |
| 3s | 21.843 | 22.664 | 23.552 | 24.362 | 25.172 | 25.982 | 26.792 | 27.601 | 28.439 | 29.221 | 30.031 | 30.841 | 31.631 | 32.420 | 33.209 | 33.998 | 34.787 | 35.576 |
| 3p | 21.303 | 22.168 | 23.093 | 23.846 | 24.616 | 25.474 | 26.384 | 27.221 | 28.154 | 29.020 | 29.809 | 30.692 | 31.521 | 32.353 | 33.184 | 34.009 | 34.841 | 35.668 |
| 4s | 12.388 | 13.444 | 14.264 | 14.902 | 15.283 | 16.096 | 17.198 | 17.656 | 18.582 | 18.986 | 19.865 | 20.869 | 21.761 | 22.658 | 23.544 | 24.408 | 25.297 | 26.173 |
| 3d | 21.679 | 22.726 | 25.397 | 25.567 | 26.247 | 27.228 | 28.353 | 29.359 | 30.405 | 31.451 | 32.540 | 33.607 | 34.678 | 35.742 | 36.800 | 37.839 | 38.901 | 39.947 |
| 4p | 10.881 | 11.932 | 12.746 | 13.460 | 14.084 | 14.977 | 15.811 | 16.435 | 17.140 | 17.723 | 18.562 | 19.411 | 20.369 | 21.265 | 22.181 | 23.122 | 24.030 | 24.957 |
| 5s | 4.985 | 6.071 | 6.256 | 6.446 | 5.921 | 6.106 | 7.227 | 6.485 | 6.640 | (empty) | 6.756 | 8.192 | 9.512 | 10.629 | 11.617 | 12.538 | 13.404 | 14.218 |
| 4d | 15.958 | 13.072 | 11.238 | 11.392 | 12.882 | 12.813 | 13.442 | 13.618 | 14.763 | 15.877 | 16.942 | 17.970 | 18.974 | 19.960 | 20.934 | 21.893 | ||
| 5p | 8.470 | 9.102 | 9.995 | 10.809 | 11.612 | 12.425 | ||||||||||||
See also
- Atomic orbitals
- Core charge
- d-block contraction (or scandide contraction)
- Electronegativity
- Lanthanide contraction
- Shielding effect
- Slater-type orbitals
- Valence electrons
- Weak charge
References
- Clementi, E.; Raimondi, D. L. (1963). "Atomic Screening Constants from SCF Functions". J. Chem. Phys. 38 (11): 2686–2689. Bibcode:1963JChPh..38.2686C. doi:10.1063/1.1733573.
- Clementi, E.; Raimondi, D. L.; Reinhardt, W. P. (1967). "Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons". Journal of Chemical Physics. 47: 1300–1307. Bibcode:1967JChPh..47.1300C. doi:10.1063/1.1712084.
Resources
- Brown, Theodore; LeMay, H.E.; & Bursten, Bruce (2002). Chemistry: The Central Science (8th revised edition). Upper Saddle River, New Jersey 07458: Prentice-Hall. ISBN 0-13-061142-5.