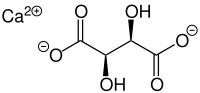
Calcium tartrate
Calcium tartrate, exactly calcium L-tartrate, is a byproduct of the wine industry, prepared from wine fermentation dregs. It is the calcium salt of L-tartaric acid, an acid most commonly found in grapes. Its solubility decreases with lower temperature, which results in the forming of whitish (in red wine often reddish) crystalline clusters as it precipitates. As E number E354, it finds use as a food preservative and acidity regulator. Like tartaric acid, calcium tartrate has two asymmetric carbons, hence it has two chiral isomers and a non-chiral isomer (meso-form). Most calcium tartrate of biological origin is the chiral levorotatory (–) isomer.
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Dihydroxybutanedioic acid calcium salt | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.656 |
| EC Number |
|
| E number | E354 (antioxidants, ...) |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CaC4H4O6 | |
| Molar mass | 190.16484 g/mol (anhydrous) 260.21 g/mol (tetrahydrate) |
| Appearance | hygroscopic white powder or colorless crystals |
| Density | 1.817 g/cm3 (tetrahydrate) |
| Melting point | tetrahydrate decomposes at 160 °C anhydrous decomposes at 650 °C |
| 0.037 g/100 ml (0 °C) 0.2 g/100 ml (85 °C) | |
| Structure | |
| d or l rhombic dl triclinic | |
| Hazards | |
| Safety data sheet | Calcium tartrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.