Calcium propanoate
Calcium propanoate or calcium propionate has the formula Ca(C2H5COO)2. It is the calcium salt of propanoic acid.
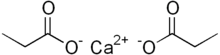 | |
| Names | |
|---|---|
| IUPAC name
Calcium propanoate | |
| Other names
Calcium propionate Calcium dipropionate Mycoban | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.021.633 |
| EC Number |
|
| E number | E282 (preservatives) |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10CaO4 | |
| Molar mass | 186.2192 g/mol |
| Appearance | White crystalline solid |
| 49 g/100 mL (0 °C) 55.8 g/100 mL (100 °C) | |
| Solubility | slightly soluble in methanol, ethanol insoluble in acetone, benzene |
| Structure | |
| monoclinic | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
As a food additive, it is listed as E number 282 in the Codex Alimentarius. Calcium propionate is used as a preservative in a wide variety of products, including but not limited to: bread, other baked goods, processed meat, whey, and other dairy products.[2] In agriculture, it is used, amongst other things, to prevent milk fever in cows and as a feed supplement [3] Propionates prevent microbes from producing the energy they need, like benzoates do. However, unlike benzoates, propionates do not require an acidic environment.[4]
Calcium propionate is used in bakery products as a mold inhibitor, typically at 0.1-0.4% [5] (though animal feed may contain up to 1%). Mold contamination is considered a serious problem amongst bakers, and conditions commonly found in baking present near-optimal conditions for mold growth.[6]
A few decades ago, Bacillus mesentericus (rope), was a serious problem,[7] but today's improved sanitary practices in the bakery, combined with rapid turnover of the finished product, have virtually eliminated this form of spoilage. Calcium propionate and sodium propionate are effective against both B. mesentericus rope and mold.[8]
Metabolism of propionate begins with its conversion to propionyl coenzyme A (propionyl-CoA), the usual first step in the metabolism of carboxylic acids. Since propanoic acid has three carbons, propionyl-CoA can directly enter neither beta oxidation nor the citric acid cycles. In most vertebrates, propionyl-CoA is carboxylated to D-methylmalonyl-CoA, which is isomerised to L-methylmalonyl-CoA. A vitamin B12-dependent enzyme catalyzes rearrangement of L-methylmalonyl-CoA to succinyl-CoA, which is an intermediate of the citric acid cycle and can be readily incorporated there.
Children were challenged with calcium propionate or placebo through daily bread in a double‐blind placebo‐controlled crossover trial. Although there was no significant difference by two measures, a statistically significant difference was found in the proportion of children whose behaviours "worsened" with challenge (52%), compared to the proportion whose behaviour "improved" with challenge (19%).[9] When propanoic acid was infused directly into rodents' brains, it produced reversible behavior changes (e.g. hyperactivity, dystonia, social impairment, perseveration) and brain changes (e.g. innate neuroinflammation, glutathione depletion) partially mimicking human autism.[10]
Calcium propionate can be used as a fungicide on fruit.[11]
In a 1973 study reported by the EPA, the waterborne administration of 180 ppm of calcium propionate was found to be slightly toxic to bluegill sunfish.[12]
Banning
Calcium propanoate has been banned in certain countries such as Russia, France and China due to certain allergies and bloating.
References
- Merck Index, 11th Edition, 1705.
- Codex Alimentarius data for calcium propanoate Archived 2006-10-21 at the Wayback Machine
- Center for Food and Nutrition Policy review of use of calcium propanoate as an organic agent in cow feed and as milk fever prevention
- "Ingredients -- Calcium propionate". Retrieved 2007-03-10.
- "Archived copy". Archived from the original on 2010-04-12. Retrieved 2010-02-28.CS1 maint: archived copy as title (link)
- "Keeping molds, bacteria at bay". Retrieved 2007-03-24.
- Furia, T. E. (1973). CRC Handbook of Food Additives. CRC Handbook of Food Additives. CRC Press.
- Furia, T. E. (1973). CRC Handbook of Food Additives. CRC Handbook of Food Additives. CRC Press.
- S. Dengate; A. Rubin (2002). "Controlled trial of cumulative behavioural effects of a common bread preservative". Journal of Paediatrics and Child Health. 38 (4): 373–376. doi:10.1046/j.1440-1754.2002.00009.x.
- D. F. MacFabe; D. P. Cain; K. Rodriguez-Capote; A. E. Franklin; J. E. Hoffman; F. Boon; A. R. Taylor; M. Kavaliers; K.-P. Ossenkopp (2007). "Neurobiological effects of intraventricular propionic acid in rats: Possible role of short-chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders". Behavioural Brain Research. 176 (1): 149–169. doi:10.1016/j.bbr.2006.07.025. PMID 16950524.
- Biggs, A. R.; El-Kholi, M. M.; El-Neshawy, S.; Nickerson, R. (1997). "Effects of Calcium Salts on Growth, Polygalacturonase Activity, and Infection of Peach Fruit by Monilinia fructicola". Plant Disease. 81 (4): 399. doi:10.1094/PDIS.1997.81.4.399.
- "OPP PESTICIDE ECOTOXICITY DATABASE - Details - Pesticide: Calcium propionate". EPA / USDA / NIFA. Archived from the original on 2019-01-23. Retrieved 2019-01-22.
