Doubly ionized oxygen
In astronomy and atomic physics, doubly ionized oxygen is the ion O2+ (also known as O III in spectroscopic notation). Its emission forbidden lines in the visible spectrum fall primarily at the wavelength 500.7 nm, and secondarily at 495.9 nm. Before spectra of oxygen ions became known, these lines once led to a spurious identification of the substance as a new chemical element. Concentrated levels of O III are found in diffuse and planetary nebulae. Consequently, narrow band-pass filters that isolate the 501 nm and 496 nm wavelengths of light, that correspond to green-turquoise-cyan spectral colors, are useful in observing these objects, causing them to appear at higher contrast against the filtered and consequently blacker background of space (and possibly light-polluted terrestrial atmosphere) where the frequencies of [O III] are much less pronounced.
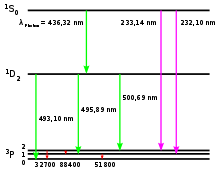
These emission lines were first discovered in the spectra of planetary nebulae in the 1860s. At that time, they were thought to be due to a new element which was named nebulium. In 1927, Ira Sprague Bowen published the current explanation identifying their source as doubly ionized oxygen.[1]
Permitted lines of O III lie in the Middle Ultraviolet band and are hence inaccessible to terrestrial astronomy.
See also
References
- Bowen, I. S. (1927). "The Origin of the Nebulium Spectrum". Nature. 120 (3022): 473. Bibcode:1927Natur.120..473B. doi:10.1038/120473a0.