Dirhenium decacarbonyl
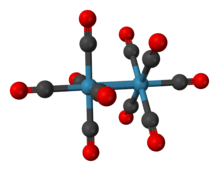
Dirhenium decacarbonyl is the inorganic compound with the chemical formula Re2(CO)10 . Commercially available, it is used as a starting point for the synthesis of many rhenium carbonyl complexes. It was first reported in 1941 by Walter Hieber, who prepared it by reductive carbonylation of rhenium.[1] The compound consists of a pair of square pyramidal Re(CO)5 units joined via a Re-Re bond, which produces a homoleptic carbonyl complex.[2]
 | |
| Names | |
|---|---|
| IUPAC name
bis(pentacarbonylrhenium)(Re—Re) | |
| Other names
Rhenium carbonyl; rhenium pentacarbonyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.714 |
PubChem CID |
|
| |
| |
| Properties | |
| Re2(CO)10 | |
| Molar mass | 652.52 g/mol |
| Melting point | 170 °C (decomposes) |
| Hazards | |
EU classification (DSD) (outdated) |
Harmful (Xn) |
| R-phrases (outdated) | R20 |
| S-phrases (outdated) | S36 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
In the 1930s Robert Mond developed methods which used increased pressure and temperature to produce various forms of metal carbonyl . A prominent scientist of the twentieth century, Walter Hieber was crucial to the further development of specifically the dirhenium decacarbonyl. Initial efforts produced mononuclear metal complexes, but upon further evaluation, Hieber discovered that by using Re2O7 as a starting material with no solvent, a dirhenium complex could be achieved producing a Re-Re interaction.[3]
Structure and properties
The crystal structure of Re2(CO)10 is relatively well known. The compound consists of a pair of square pyramidal Re(CO)5 units linked by a Re-Re bond. There are two different conformations that can occur: staggered and eclipsed. The eclipsed conformation occurs about 30% of the time, producing a D4h point group, but the staggered form, with point group D4d, is more stable. The Re-Re bond length was experimentally found to be 3.04Å.[4]
The Re atom exists in a slightly distorted octahedral configuration with the C axial-Re-C equatorial angle equal to 88°. The mean Re-C bond length of 2.01 Å is the same for the axial and equatorial positions. The mean C-O distance is 1.16 Å.[1][5]
This compound has a broad IR absorption band at 1800 cm−1 region can be assigned to two components centered at 1780 and 1830 cm−1, resulting from CO adsorption. The remaining nine CO groups in Re2(CO)10 give the complex IR absorption in the 1950–2150 cm−1 region. Free Re2(CO)10 (point symmetry D4d ) has a CO stretch representation of 2A1+E2 + E3+ 2B2 +E1, where 2B2 + E1 are IR active. For an axially perturbed (C4v) Re2(CO)10 molecule, the CO stretch representation was found to be 2E+B1+B2+3A1, where the IR active modes are 2E+3A1.[6]
Its identity can also be confirmed by mass spectrometry, using the isotopic pattern of rhenium (185Re and 187Re).[7]
Synthesis
Dirhenium decacarbonyl may be obtained by reductive carbonylation of rhenium(VII) oxide (Re2O7) at 350 atm and 250 °C.[3]
- Re2O7 + 17 CO → Re2(CO)10 + 7 CO2
Reactions
The carbonyl ligands may be displaced by other ligands such as phosphines and phosphites (denoted L).[7][8]
- Re2(CO)10 + 2 L → Re2(CO)8L2
This compound may also be "cracked" to mononuclear Re(I) carbonyl complexes by halogenation:[9]
- Re2(CO)10 + X2 → 2 Re(CO)5X (X = Cl, Br, I)
When bromine is used, bromopentacarbonylrhenium(I) is formed, which is an intermediate for many more rhenium complexes.[7] This compound may also be hydrogenated to form various polyrhenium complexes, eventually giving elemental rhenium.[10]
- Re2(CO)10 → H3Re3(CO)12 → H5Re4(CO)12 → Re (metal)
In the presence of water, photolysis of Re2(CO)10 yields a hydroxide complex:[11]
- Re2(CO)10 → HRe(CO)5 + Re4(CO)12(OH)4
This reaction includes the cleavage of Re-Re bond and the synthesis of HRe(CO)5, which can be used to prepare surface structures designed to incorporate isolated surface-bound Re carbonyl complexes.[12]
Loss of a carbonyl ligand by photolysis generates a coordinatively unsaturated complex that undergoes oxidative addition of Si-H bonds, for example:
- Re2(CO)10 + HSiCl3* → (CO)5ReHRe(CO)4SiCl3 + CO
Applications
Rhenium-based catalysis have been used in metathesis, reforming, hydrogenation and various hydrotreating processes such as hydrodesulfurization.[13] Re2(CO)10 can be used to promote the silation of alcohols and prepare the silyl ethers, and its reaction:[14]
- RSiH3 + R’OH → RH2SiOR’ + H2
See also
References
- W. Hieber; H. Fuchs (1941). "Über Metallcarbonyle. XXXVIII. Über Rheniumpentacarbonyl". Zeitschrift für anorganische und allgemeine Chemie (in German). 248 (3): 256–268. doi:10.1002/zaac.19412480304.
- F. Armstrong; J. Rourke; M. Hagerman; M. Weller; P. Atkins; T. Overton (2010). "Shiver and Atkins' Inorganic Chemistry 5th edition": 555. Cite journal requires
|journal=(help) - H. Werner (2009). "Organo-Transition Metal Chemistry: A personal View": 93. Cite journal requires
|journal=(help) - M. Churchill; K. Amoh; H. Wasserman (1981). "Redetermination of the crystal structure of dimanganese decacarbonyl and determination of the crystal structure of dirhenium decacarbonyl. Revised values for the manganese-manganese and rhenium-rhenium bond lengths in dimanganese decacarbonyl and dirhenium decacarbonyl". Inorganic Chemistry. 20 (3): 1609–1612. doi:10.1021/ic50219a056.
- N.IGapotchenko; et al. (1972). "Molecular structure of dirhenium decacarbonyl". Journal of Organometallic Chemistry. 35 (2): 319–320. doi:10.1016/S0022-328X(00)89806-X.
- E. Escalona Platero; F.R. Peralta; C. Otero Areán (1995). "Vapour phase deposition and thermal decarbonylation of Re2(CO)10 on gamma-alumina: infrared studies". Catalysis Letters. 34 (1): 65–73. doi:10.1007/BF00808323.
- A.M. Stolzenberg; E.L. Muetterties (1983). "Mechanisms of dirhenium decacarbonyl substitution reactions: crossover experiments with dirhenium-185 decacarbonyl and dirhenium-187 decacarbonyl". Journal of the American Chemical Society. 105 (4): 822–827. doi:10.1021/ja00342a029.
- K.S. Suslick; P.F. Schubert (1983). "Sonochemistry of dimanganese decacarbonyl (Mn2(CO)10) and dirhenium decacarbonyl (Re2(CO)10)". Journal of the American Chemical Society. 105 (19): 6042–6044. doi:10.1021/ja00357a014.
- Steven P. Schmidt; William C. Trogler; Fred Basolo (2007). Pentacarbonylrhenium Halides. Inorganic Syntheses. 28. pp. 154–159. doi:10.1002/9780470132593.ch42. ISBN 9780470132593.
- C. Dossi, J. Schaefer, W. M. H. Sachtler (1989). "Mechanism of particle formation in decomposing Re2(CO)10 on NaY and NaHY zeolites: effect of prereduced Pt clusters in the supercages". Journal of Molecular Catalysis. 52 (1): 193–209. doi:10.1016/0304-5102(89)80089-6.CS1 maint: multiple names: authors list (link)
- D. R. Gard; T. L. Brown (1982). "Photochemical reactions of dirhenium decacarbonyl with water". Journal of the American Chemical Society. 104 (23): 6340–6347. doi:10.1021/ja00387a031.
- P. S. Kirlin; et al. (1990). "Surface catalytic sites prepared from [HRe(CO)5] and [H3Re3(CO)12]: mononuclear, trinuclear, and metallic rhenium catalysts supported on magnesia". Journal of Physical Chemistry. 94 (92): 8439–8450. doi:10.1021/j100385a017. hdl:1874/5964.
- R. Jarkko; A.P. Tapani (2000). "Controlled gas‐phase preparation and HDS activity of Re2(CO)10 alumina catalysts". Catalysis Letters. 65 (4): 175–180. doi:10.1023/A:1019006413873.
- D.H.R.Barton, M.J. Kelly (1992). "Mechanism and utility of the dirhenium decacarbonyl catalyzed formation of silyl ethers". Tetrahedron Letters. 33 (35): 5041–5044. doi:10.1002/chin.199302225.