Dipropylene glycol
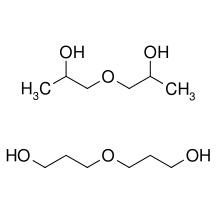
Dipropylene glycol is a mixture of three isomeric chemical compounds, 4-oxa-2,6-heptandiol, 2-(2-hydroxy-propoxy)-propan-1-ol, and 2-(2-hydroxy-1-methyl-ethoxy)-propan-1-ol. It is a colorless, nearly odorless liquid with a high boiling point and low toxicity.[2][3]
 | |
| Names | |
|---|---|
| IUPAC names
4-Oxa-2,6-heptandiol and 4-Oxa-1,6-heptandiol | |
| Other names
1,1'-Oxybis(1-propanol) and 1,1'-Oxybis(2-propanol) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.042.504 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H14O3 | |
| Molar mass | 134.173 g/mol |
| Appearance | colorless liquid |
| Density | 1.0206 g/cm3 at 20 °C |
| Boiling point | 230.5 °C (446.9 °F; 503.6 K)[1] |
| miscible with water, soluble in ethanol | |
| Hazards | |
| Safety data sheet | SIRI.org |
| NFPA 704 (fire diamond) | |
| Flash point | 121 °C (250 °F; 394 K) |
| 310 °C (590 °F; 583 K) | |
| Related compounds | |
Related compounds |
Ethylene glycol Propylene glycol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Dipropylene glycol finds many uses as a plasticizer, an intermediate in industrial chemical reactions, as a polymerization initiator or monomer, and as a solvent. Its low toxicity and solvent properties make it an ideal additive for perfumes and skin and hair care products. It is also a common ingredient in commercial fog fluid, used in entertainment industry fog machines.[2][3][4]
gollark: What do you mean?
gollark: "Modular" laptops.
gollark: People seem weirdly interested in Framework given that they are still ridiculously tightly integrated, more so than even Haswell-era laptops with their PGA things.
gollark: If this happens right after my computers all implode, guess I just lose access to everything.
gollark: Under normal circumstances, I just buy a new one and copy data back on.
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. p. 342. ISBN 0-8493-0594-2.
- "Dipropylene Glycol Regular Grade (DPG)". Dow Chemical. Archived from the original on 2009-03-15. Retrieved 2009-04-07.
- Lloyd R. Whittington, ed. (1993). Whittington's Dictionary of Plastics (3 ed.). Technomic Publishing. p. 138. ISBN 1-56676-090-9. Retrieved 2009-04-07.
- "Dipropylene Glycol LO+ (DPG LO+)". Dow Chemical. Archived from the original on 2009-06-19. Retrieved 2009-04-07.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
